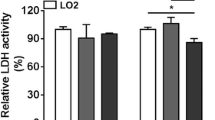
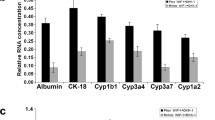
Abstract
Background and Aims
Bioartificial livers (BALs) have attracted much attention as potential supportive therapies for liver diseases. A serum-free microcarrier culture strategy for the in vitro high-density expansion of human-induced hepatocyte-like cells (hiHeps) suitable for BALs was studied in this article.
Methods
hiHeps were transdifferentiated from human fibroblasts by the lentiviral overexpression of FOXA3, HNF1A, and HNF4A. Cells were cultured on microcarriers, their proliferation was evaluated by cell count and CCK-8 assays, and their function was evaluated by detecting liver function parameters in the supernatant, including urea secretion, albumin synthesis, and lactate dehydrogenase levels. The expressions of hepatocyte function-associated genes of hiHeps were measured by qRT-PCR in 2D and 3D conditions. The expression of related proteins during fibronectin promotes cell adhesion, and proliferation on microcarrier was detected by western blotting.
Results
During microcarrier culture, the optimal culture conditions during the adherence period were the use of half-volume high-density inoculation, Cytodex 3 at a concentration of 3 mg/mL, a cell seeding density of 2.0 × 105 cells/mL, and a stirring speed of 45 rpm. The final cell density in self-developed, chemically defined serum-free medium (SFM) reached 2.53 × 106 cells/mL, and the maximum increase in expansion was 12.61-fold. In addition, we found that fibronectin (FN) can promote hiHep attachment and proliferation on Cytodex 3 microcarriers and that this pro-proliferative effect was mediated by the integrin-β1/FAK/ERK/CyclinD1 signaling pathway. Finally, the growth and function of hiHeps on Cytodex 3 in SFM were close to those of hiHeps on Cytodex 3 in hepatocyte maintenance medium (HMM), and cells maintained their morphology and function after harvest on microcarriers.
Conclusions
Serum-free microcarrier culture has important implications for the expansion of a sufficient number of hiHeps prior to the clinical application of BALs.






Similar content being viewed by others
References
Martin P, Friedman LS. Assessment of liver function and diagnostic studies. In: Handbook of Liver Disease. 2018:1–17.
Hernaez R, Solà E, Moreau R, et al. Acute-on-chronic liver failure: an update. Gut. 2017;66:541–553.
Habib S, Shaikh OS. Drug-induced acute liver failure. Clin Liver Dis. 2017;21(1):151–162.
Bernal W, Wendon J. Acute liver failure. N Engl J Med. 2013;369:2525–2534.
Struecker B, Raschzok N, Sauer IM. Liver support strategies: cutting-edge technologies. Nat Rev Gastroenterol Hepatol. 2013;11:166–176.
Sussman NL, Kelly JH. Artificial liver. Clin Gastroenterol Hepatol. 2014;12:1439–1442.
Lee KCL, Stadlbauer V, Jalan R. Extracorporeal liver support devices for listed patients. Liver Transpl. 2016;22(6):839–48.
Yu CB, Pan XP, Li LJ. Progress in bioreactors of bioartificial livers. Hepatobiliary Pancreat Dis Int. 2009;8:134–140.
Gu J, Shi X, Ren H, et al. Systematic review: extracorporeal bio-artificial liver-support system for liver failure. Hep Intl. 2012;6:670–683.
Pan XP, Li LJ. Advances in cell sources of hepatocytes for bioartificial live. Hepatobiliary Pancreat Dis Int. 2012;11:594–605.
Huang P, He Z, Ji S, et al. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. 2011;475:386–389.
Huang P, Zhang L, Gao Y, et al. Direct reprogramming of human fibroblasts to functional and expandable hepatocytes. Cell Stem Cell. 2014;14:370–384.
Shi XL, Gao Y, Yan Y, et al. Improved survival of porcine acute liver failure by a bioartificial liver device implanted with induced human functional hepatocytes. Cell Res. 2016;26:206.
Valk JVD, Brunner D, Smet KD, et al. Optimization of chemically defined cell culture media—replacing fetal bovine serum in mammalian in vitro methods. Toxicol In Vitro. 2010;24:1053–1063.
Gu C, Li PP, Liu W, et al. The role of insulin in transdifferentiated hepatocyte proliferation and function in serum-free medium. J Cell Mol Med. 2019;23:4165–4178.
Blüml Gerald. Microcarrier cell culture technology. Methods Biotechnol. 2007;24:149–178.
by Vero Cells Grown on Cytodex 1 Microcarriers in a 2-Litre Stirred Tank Bioreactor. J Biomed Biotechnol. 2015;2010:586363.
Fernandes AM, Fernandes TG, Diogo MM, et al. Mouse embryonic stem cell expansion in a microcarrier-based stirred culture system. J Biotechnol. 2007;132:227–236.
Tao X, Shaolin L, Yaoting Y. Preparation and culture of hepatocyte on gelatin microcarriers. J Biomed Mater Res, Part A. 2010;65:306–310.
Schulz CM, Ruzicka J. Real-time determination of glucose consumption by live cells using a lab-on-valve system with an integrated microbioreactor. The Analyst. 2002;127:1293–1298.
Werner A, Duvar S, Müthing Johannes, et al. Cultivation of immortalized human hepatocytes HepZ on macroporous CultiSpher G microcarriers. Biotechnol Bioeng. 2000;68:59–70.
Gstraunthaler G, Lindl T, et al. A plea to reduce or replace fetal bovine serum in cell culture media. Cytotechnology. 2013;65:791–793.
Li PP, Gu C, Liang BY, et al. A serum-free medium suitable for maintaining cell morphology and liver-specific function in induced human hepatocytes. Cytotechnology. 2019;71:329–344.
Dohi N, Takahashi T, Minekawa K, et al. Power consumption and solid suspension performance of large-scale impellers in gas–liquid–solid three-phase stirred tank reactors. Chem Eng J. 2004;97:103–114.
Frijlink JJ, Bakker A, Smith JM. Suspension of solid particles with gassed impellers. Chem Eng Sci. 1990;45:1703–1718.
Croughan MS, Hamel JF, Wang DIC. Hydrodynamic effects on animal cells grown in microcarrier cultures. Biotechnol Bioeng. 2000;67:841–852.
Jiang D, Hu J, Zhou Y, et al. Optimization of attachment conditions for rabbit mesenchymal stem cells in cytodex 3 microcarrier culture systems. Shengwu yixue gongchengxue zazhi. 2007;24:884.
Shiojiri N, Sugiyama Y. Immunolocalization of extracellular matrix components and integrins during mouse liver development. Hepatology. 2004;40:346–355.
Nienow AW, Hewitt CJ, Heathman TRJ, et al. Agitation conditions for the culture and detachment of hMSCs from microcarriers in multiple bioreactor platforms. Biochem Eng J. 2016;108:24–29.
Burnouf T, Griffiths E, Padilla A, et al. Assessment of the viral safety of antivenoms fractionated from equine plasma. Biologicals. 2004;32:115–128.
Yi G, Huanzhang H, Ke C, et al. Primary porcine hepatocytes with portal vein serum cultured on microcarriers or in spheroidal aggregates. World J Gastroenterol. 2000;6:365–370.
Yiheng C, Shuyu T, Xuping L, et al. The effects of microcarrier concentration and cell density on the growth of swine testicle cells. Biotechnol Bull. 2016;32:242–250.
Demetriou AA, Reisner A, Sanchez J, et al. Transplantation of microcarrier-attached hepatocytes into 90% partially hepatectomized rats. Hepatology (Baltimore, Md.),. 1988;8:1006–1009.
Hewitt CJ, Lee K, Nienow AW, et al. Expansion of human mesenchymal stem cells on microcarriers[J]. Biotech Lett. 2011;33:2325–2335.
Shin WY, Lee KU, Lee HW, et al. Optimal number of hepatocytes per microcarrier in spheroid culture using cytodex 3 microcarrier. J Korean Surg Soc. 2007;73:235–241.
Liu ML, Mars WM, Zarnegar R, et al. Collagenase pretreatment and the mitogenic effects of hepatocyte growth factor and transforming growth factor-alpha in adult rat liver. Hepatology. 2010;19:1521–1527.
Grinnell F, Hays DG, Minter D. Cell adhesion and spreading factor: partial purification and properties. Exp Cell Res. 1977;110:175–190.
Hughes RC, Pena SDJ, Clark J, et al. Molecular requirements for the adhesion and spreading of hamster fibroblasts. Exp Cell Res. 1979;121:307–314.
Feinberg AW, Schumacher JF, Brennan AB. Engineering high-density endothelial cell monolayers on soft substrates. Acta Biomater. 2009;5:2013–2024.
Matsuo M, Sakurai H, Ueno Y, et al. Activation of MEK/ERK and PI3K/Akt pathways by fibronectin requires integrin αv-mediated ADAM activity in hepatocellular carcinoma: a novel functional target for gefitinib. Cancer Sci. 2006;97:155–162.
Illario M, Cavallo AL, Monaco S, et al. Fibronectin-induced proliferation in thyroid cells is mediated by αvβ3 integrin through Ras/Raf-1/MEK/ERK and calcium/CaMKII signals. J Clin Endocrinol Metab. 2005;90:2865–2873.
Gigout A, Buschmann MD, Jolicoeur M. Chondrocytes cultured in stirred suspension with serum-free medium containing pluronic-68 aggregate and proliferate while maintaining their differentiated phenotype. Tissue Eng Part A. 2009;15:2237–2248.
Huang L, Xiao L, Jung Poudel A, et al. Porous chitosan microspheres as microcarriers for 3D cell culture. Carbohyd Polym. 2018;202:611–620.
Rebelo SP, Costa R, Silva MM, et al. Three-dimensional co-culture of human hepatocytes and mesenchymal stem cells: improved functionality in long-term bioreactor cultures. J Tissue Eng Regen Med. 2017;11:2034–2045.
Wei G, Wang J, Lv Q, et al. Three-dimensional coculture of primary hepatocytes and stellate cells in silk scaffold improves hepatic morphology and functionality in vitro. J Biomed Mater Res, Part A. 2018;106:2171.
Acknowledgments
We thank the Shanghai Institutes for Biological Sciences of the Chinese Academy of Sciences for providing hiHeps and the cell culturing protocol. Ce Gu performed all the experiments and wrote the manuscript. Miaomiao Chai, Jiaxing Liu, Hui Wang, and Wenjing Du were involved in useful discussions during the development of this study. Yan Zhou and Wen-Song Tan contributed to the conception, design of the work or of parts of it, and its interpretation.
Funding
This research was supported by the Basic Research Project of Shanghai Science and Technology Commission (Grant No. 16JC1400203), the National Key Research and Development Program of China, 2018YFC1105801, and the National Natural Science Foundation of China (Grant No. 81671841).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gu, C., Chai, M., Liu, J. et al. Expansion of Transdifferentiated Human Hepatocytes in a Serum-Free Microcarrier Culture System. Dig Dis Sci 65, 2009–2023 (2020). https://doi.org/10.1007/s10620-019-05925-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05925-8