Abstract
Background
Helicobacter pylori (H. pylori) eradication can reduce the prevalence of gastric cancer. However, whether H. pylori eradication therapy should be performed in infected patients, especially in asymptomatic cases, is still controversial.
Aims
The aims of this study were to determine whether H. pylori screening and eradication could prevent gastric cancer in a cost-effective way, and further whether eradication therapy should be administered to asymptomatic individuals.
Methods
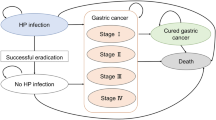
Cost-effectiveness analysis was performed using a Markov model. We established two groups, each with 10,000 hypothetical Chinese individuals at the age of 40 years. Clinical outcomes and cost of H. pylori eradication were compared between the eradication and control groups.
Results
There was a lower morbidity with gastric cancer in the eradication group than in the control group, which was most significant after running the model for 15 years. The eradication group experienced an average of 34.64 quality-adjusted life years (QALYs) per person, and the average cost was US $1706.52 per person. The control group exhibited an average of 32.63 QALYs per person, and the average cost was US $2045.10 per person. The cost-effectiveness analysis showed that eradication saved $1539 per LY per person and $168.45 per QALY per person.
Conclusions
H. pylori screening and eradication therapy effectively reduces the morbidity of gastric cancer and cancer-related costs in asymptomatic infected individuals. Therefore, we believe that H. pylori eradication can prevent gastric cancer in a cost-effective way.





Similar content being viewed by others
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386.
Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044.
Forman D, Sitas F, Newell DG, et al. Geographic association of Helicobacter pylori antibody prevalence and gastric cancer mortality in rural China. Int J Cancer. 1990;46:608–611.
Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7–14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241.
Lahat A, Kopylov U, Neuman S, et al. Helicobacter pylori prevalence and clinical significance in patients with quiescent Crohn’s disease. BMC Gastroenterol. 2017;17:27.
Sipponen P. Natural history of gastritis and its relationship to peptic ulcer disease. Digestion. 1992;51:70–75.
Moayyedi P, Forman D, Braunholtz D, et al. The proportion of upper gastrointestinal symptoms in the community associated with Helicobacter pylori, lifestyle factors, and nonsteroidal anti-inflammatory drugs. Leeds HELP Study Group. Am J Gastroenterol. 2000;95:1448–1455.
Sugano K. Screening of gastric cancer in Asia. Best Pract Res Clin Gastroenterol. 2015;29:895–905.
Sonnenberg A, Lash RH, Genta RM. A national study of Helicobacter pylori infection in gastric biopsy specimens. Gastroenterology. 2010;139:1894–1901.
Warren JR. Gastric pathology associated with Helicobacter pylori. Gastroenterol Clin North Am. 2000;29:705–751.
Zhou Q, Li L, Ai Y, Pan Z, Guo M, Han J. Diagnostic accuracy of the (14)C-urea breath test in Helicobacter pylori infections: a meta-analysis. Wien Klin Wochenschr. 2017;129:38–45.
The EUROGAST Study Group. Epidemiology of, and risk factors for, Helicobacter pylori infection among 3194 asymptomatic subjects in 17 populations. Gut. 1993;34:1672–1676.
Helicobacter, Cancer Collaborative G. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49:347–353.
Bae SE, Choi KD, Choe J, et al. The effect of eradication of Helicobacter pylori on gastric cancer prevention in healthy asymptomatic populations. Helicobacter. 2018;23:e12464.
Wong BC, Lam SK, Wong WM, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187–194.
Ford AC, Forman D, Hunt RH, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ. 2014;348:g3174.
Correa P, Fontham ET, Bravo JC, et al. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-Helicobacter pylori therapy. J Natl Cancer Inst. 2000;92:1881–1888.
Ma JL, Zhang L, Brown LM, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst. 2012;104:488–492.
Wong BC, Zhang L, Ma JL, et al. Effects of selective COX-2 inhibitor and Helicobacter pylori eradication on precancerous gastric lesions. Gut. 2012;61:812–818.
Zhou L, Lin S, Ding S, et al. Relationship of Helicobacter pylori eradication with gastric cancer and gastric mucosal histological changes: a 10-year follow-up study. Chin Med J (Engl). 2014;127:1454–1458.
Xie F, Luo N, Lee HP. Cost effectiveness analysis of population-based serology screening and (13)C-urea breath test for Helicobacter pylori to prevent gastric cancer: a Markov model. World J Gastroenterol. 2008;14:3021–3027.
Park YM, Kim JH, Baik SJ, Park JJ, Youn YH, Park H. Clinical risk assessment for gastric cancer in asymptomatic population after a health check-up: an individualized consideration of the risk factors. Medicine (Baltimore). 2016;95:e5351.
Shin DW, Yun YH, Choi IJ, Koh E, Park SM. Cost-effectiveness of eradication of Helicobacter pylori in gastric cancer survivors after endoscopic resection of early gastric cancer. Helicobacter. 2009;14:536–544.
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132.
Schlesinger-Raab A, Mihaljevic AL, Egert S, et al. Outcome of gastric cancer in the elderly: a population-based evaluation of the Munich Cancer Registry. Gastric Cancer. 2016;19:713–722.
Yan TL, Hu QD, Zhang Q, Li YM, Liang TB. National rates of Helicobacter pylori recurrence are significantly and inversely correlated with human development index. Aliment Pharmacol Ther. 2013;37:963–968.
Alboraie M, Saad M, Al-Ali J, et al. Quadruple therapy versus standard triple therapy for eradication of Helicobacter pylori in Kuwait. Arab J Gastroenterol. 2015;16:131–135.
Song Z, Zhou L, Zhang J, He L, Bai P, Xue Y. Levofloxacin, bismuth, amoxicillin and esomeprazole as second-line Helicobacter pylori therapy after failure of non-bismuth quadruple therapy. Dig Liver Dis. 2016;48:506–511.
Wang L, Lin Z, Chen S, et al. Ten-day bismuth-containing quadruple therapy is effective as first-line therapy for Helicobacter pylori-related chronic gastritis: a prospective randomized study in China. Clin Microbiol Infect. 2017;23:391–395.
Pan KF, Zhang L, Gerhard M, et al. A large randomised controlled intervention trial to prevent gastric cancer by eradication of Helicobacter pylori in Linqu County, China: baseline results and factors affecting the eradication. Gut. 2016;65:9–18.
Wang Q, Lin GW, Xu SR. The measurement of health utility value in chronic gastritis, peptic ulcers and gastric cancer patient. Chin J Dig. 2017;23:273–274.
Lee YC, Chen TH, Chiu HM, et al. The benefit of mass eradication of Helicobacter pylori infection: a community-based study of gastric cancer prevention. Gut. 2013;62:676–682.
Kowada A. Cost-effectiveness of Helicobacter pylori screening followed by eradication treatment for employees in Japan. Epidemiol Infect. 2018;146:1834–1840.
Mason J, Axon AT, Forman D, et al. The cost-effectiveness of population Helicobacter pylori screening and treatment: a Markov model using economic data from a randomized controlled trial. Aliment Pharmacol Ther. 2002;16:559–568.
Zamani M, Ebrahimtabar F, Zamani V, et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther. 2018;47:868–876.
Wang Q, Jin PH, Lin GW, Xu SR, Chen J. Cost-effectiveness of Helicobacter pylori screening to prevent gastric cancer: Markov decision analysis. Zhonghua Liu Xing Bing Xue Za Zhi. 2003;24:135–139.
Darwin E, Murni AW, Nurdin AE. The effect of psychological stress on mucosal IL-6 and Helicobacter pylori activity in functional dyspepsia. Acta Med Indones. 2017;49:99–104.
Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer. 2015;136:487–490.
Thung I, Crowe SE, Valasek MA. Letter: global emergence of Helicobacter pylori antibiotic resistance—unanswered questions. Aliment Pharmacol Ther. 2016;43:1249–1250.
Lin KD, Chiu GF, Waljee AK, et al. Effects of anti-Helicobacter pylori therapy on incidence of autoimmune diseases, including inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2019;17:1991–1999.
Zhou X, Wu J, Zhang G. Association between Helicobacter pylori and asthma: a meta-analysis. Eur J Gastroenterol Hepatol. 2013;25:460–468.
Leung WK, Lin SR, Ching JY, et al. Factors predicting progression of gastric intestinal metaplasia: results of a randomised trial on Helicobacter pylori eradication. Gut. 2004;53:1244–1249.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81600447, 81400606), Science and Technology plan projects of Zhejiang Province (2015C33102), and Zhejiang Provincial Natural Science Foundation of China (LY17H030004).
Author information
Authors and Affiliations
Contributions
YH and LL planned and conducted the study. YH, TY, HM, CL and XY analyzed and interpreted the data. YH, TY, LL and YL drafted and revised the manuscript. All authors approved the final draft of this manuscript for submission.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Han, Y., Yan, T., Ma, H. et al. Cost-Effectiveness Analysis of Helicobacter pylori Eradication Therapy for Prevention of Gastric Cancer: A Markov Model. Dig Dis Sci 65, 1679–1688 (2020). https://doi.org/10.1007/s10620-019-05910-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05910-1