Abstract
Background
Controlled attenuation parameter (CAP) has been developed to estimate the extent of hepatic steatosis.
Aims
The purpose was to evaluate the usefulness of CAP in assessing hepatic steatosis and its change using magnetic resonance imaging proton density fat fraction (MRI-PDFF) as reference standard.
Methods
Consecutive patients with liver steatosis were enrolled prospectively. We assessed hepatic steatosis with CAP and MRI-PDFF at enrollment and 12-month follow-up. The correlations between the two methods were analyzed. With MRI-PDFF as reference, the performance of CAP in diagnosis of steatosis severity and its changes was assessed.
Results
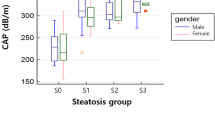
A total of 50 patients were enrolled, and 45 of them had follow-up MRI-PDFF and CAP at a median interval of 399 days. The mean hepatic steatosis was 13.4% by MRI-PDFF and 291.6 dB/m by CAP. There were positive correlations between CAP and MRI-PDFF in steatosis severity and its change. The median value of CAP was 254, 301.5, and 329.5 dB/m for steatosis < 10%, 10–20%, and > 20%, respectively. For CAP in detecting steatosis ≥ 10% and > 20%, the diagnostic performance was 0.821 and 0.814. With the cutoff of 275 dB/m for ≥ 10% steatosis, the positive predictive value was 84.8%. With the cutoff of 325 dB/m for > 20% steatosis, the negative predictive value was 91.9%. In multiple linear regression, one dB/m change by CAP was associated with 0.039% change by MRI-PDFF.
Conclusions
In assessing liver fat content, CAP correlated with MRI-PDFF and was useful for detection and monitoring of hepatic steatosis.



Similar content being viewed by others
References
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease- meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84.
Wong VW, Chan WK, Chitturi S, et al. Asia-Pacific Working Party on Nonalcoholic Fatty Liver Disease guidelines 2017-Part 1: definition, risk factors and assessment. J Gastroenterol Hepatol. 2018;33:70–85.
Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357.
European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of nonalcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402.
Bedossa P, Carrat F. Liver biopsy: the best, not the gold standard. J Hepatol. 2009;50:1–3.
Dulai PS, Sirlin CB, Loomba R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: clinical trials to clinical practice. J Hepatol. 2016;65:1006–1016.
Sasso M, Beaugrand M, de Ledinghen V, et al. Controlled attenuation parameter (CAP): a novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825–1835.
Sasso M, Tengher-Barna I, Ziol M, et al. Novel controlled attenuation parameter for noninvasive assessment of steatosis using Fibroscan(®): validation in chronic hepatitis C. J Viral Hepat. 2012;19:244–253.
Caussy C, Alquiraish MH, Nguyen P, et al. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology. 2018;67:1348–1359.
Ferraioli G, Filice C, Castera L, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 3: liver. Ultrasound Med Biol. 2015;41:1161–1179.
Hines CD, Frydrychowicz A, Hamilton G, et al. T(1) independent, T(2) (*) corrected chemical shift based fat-water separation with multi-peak fat spectral modeling is an accurate and precise measure of hepatic steatosis. J Magn Reson Imaging. 2011;33:873–881.
Noureddin M, Lam J, Peterson MR, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology. 2013;58:1930–1940.
Imajo K, Kessoku T, Honda Y, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology. 2016;150:626–637.
Karlas T, Petroff D, Sasso M, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022–1030.
Shi KQ, Tang JZ, Zhu XL, et al. Controlled attenuation parameter for the detection of steatosis severity in chronic liver disease: a meta-analysis of diagnostic accuracy. J Gastroenterol Hepatol. 2014;29:1149–1158.
Wang Y, Fan Q, Wang T, Wen J, Wang H, Zhang T. Controlled attenuation parameter for assessment of hepatic steatosis grades: a diagnostic meta-analysis. Int J Clin Exp Med. 2015;8:17654–17663.
Siddiqui MS, Vuppalanchi R, Van Natta ML, et al. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2019;17:156–163.
Wong VW, Petta S, Hiriart JB, et al. Validity criteria for the diagnosis of fatty liver by M probe-based controlled attenuation parameter. J Hepatol. 2017;67:577–584.
Karlas T, Petroff D. Is magnetic resonance imaging really more accurate for classifying steatosis than controlled attenuation parameter? Gastroenterology. 2016;151:374–375.
Tapper EB, Loomba R. Noninvasive imaging biomarker assessment of liver fibrosis by elastography in NAFLD. Nat Rev Gastroenterol Hepatol. 2018;15:274–282.
Kwok R, Choi KC, Wong GL, et al. Screening diabetic patients for nonalcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2016;65:1359–1368.
Le TA, Chen J, Changchien C, et al. Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology. 2012;56:922–932.
Papapostoli I, Lammert F, Stokes CS. Effect of short-term vitamin D correction on hepatic steatosis as quantified by controlled attenuation parameter (CAP). J Gastrointestin Liver Dis. 2016;25:175–181.
Arslanow A, Teutsch M, Walle H, Grünhage F, Lammert F, Stokes CS. Short-term hypocaloric high-fiber and high-protein diet improves hepatic steatosis assessed by controlled attenuation parameter. Clin Transl Gastroenterol. 2016;7:e176.
Ogasawara N, Kobayashi M, Akuta N, et al. Serial changes in liver stiffness and controlled attenuation parameter following direct-acting antiviral therapy against hepatitis C virus genotype 1b. J Med Virol. 2018;90:313–319.
Shimizu M, Suzuki K, Kato K, et al. Evaluation of the effects of dapagliflozin, an SGLT2 inhibitor, on hepatic steatosis and fibrosis by transient elastography in patients with type 2 diabetes and nonalcoholic fatty liver disease. Diabetes Obes Metab. 2019;21:285–292.
de Lédinghen V, Hiriart JB, Vergniol J, Merrouche W, Bedossa P, Paradis V. Controlled attenuation parameter (CAP) with the XL probe of the Fibroscan®: a comparative study with the M probe and liver biopsy. Dig Dis Sci. 2017;62:2569–2577. https://doi.org/10.1007/s10620-017-4638-3
Chan WK, Nik Mustapha NR, Mahadeva S, Wong VW, Cheng JY, Wong GL. Can the same controlled attenuation parameter cut-offs be used for M and XL probes for diagnosing hepatic steatosis? J Gastroenterol Hepatol. 2018;33:1787–1794.
Acknowledgments
We wish to thank the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital, for statistics work. This study was supported by grants (CMRPG8D1081 and CMRPG8E1291) from Chang Gung Memorial Hospital to Jing-Houng Wang.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, JH., Ou, HY., Yen, YH. et al. Usefulness of Controlled Attenuation Parameter in Detecting and Monitoring Hepatic Steatosis with MRI-PDFF as Reference. Dig Dis Sci 65, 1512–1519 (2020). https://doi.org/10.1007/s10620-019-05883-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05883-1