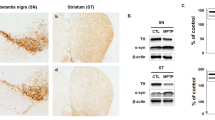
Abstract
Background
Gastrointestinal (GI) motility dysfunction is the most common non-motor symptom of Parkinson’s disease (PD). Studies have indicated that GI motility functions are impaired before the onset of PD.
Aims
To investigate the underlying mechanism of PD-induced GI dysmotility in MPTP (1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine)-induced animal model.
Methods
C57BL/6 mice were administered with or without a selective dopamine neurotoxin, MPTP, to induce parkinsonian symptoms. In addition to in vivo studies, in vitro experiments were also conducted in colon specimens using l-methyl-4-phenylpyridinium (MPP+), a metabolic product of MPTP. Gastric emptying, colon motility, nitrergic relaxation, and western blot experiments were performed as reported.
Results
MPTP-induced PD mice showed decreased expression of nuclear factor erythroid 2-related factor (Nrf2) and its target phase II genes in gastric and colon neuromuscular tissues. Decreased levels of tetrahydrobiopterin (BH4, a critical cofactor for nNOS dimerization) associated with uncoupling of nNOS in gastric and colon tissues exposed to MPTP. Impaired enteric nitrergic system led to delayed gastric emptying and slower colonic motility compared to the control mice. In vitro results in colon specimens confirm that activation of Nrf2 restored MPP+-induced suppression of alpha-synuclein, tyrosine hydroxylase (TH), Nrf2, and heme oxygenase-1. In vitro exposure to L-NAME [N(w)-nitro-l-arginine methyl ester], a NOS synthase inhibitor, reduced protein expression of TH in colon tissue homogenates.
Conclusions
Loss of Nrf2/BH4/nNOS expression in PD impairs antioxidant gene expression, which deregulates NO synthesis, thereby contributing to the development of GI dysmotility and constipation. Nitric oxide appears to be important to maintain dopamine synthesis in the colon.












Similar content being viewed by others
References
Lionnet A, Leclair-Visonneau L, Neunlist M, et al. Does Parkinsons disease start in the gut? Acta Neuropathol. 2018;135:1–12.
Natale G, Pasquali L, Ruggieri S, Paparelli A, Fornai F. Parkinson’s disease and the gut: a well known clinical association in need of an effective cure and explanation. Neurogastroenterol Motil. 2008;20:741–749.
Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease. Parkinsonism Relat Disord. 2011;17:10–15.
Anderson G, Noorian AR, Taylor G, et al. Loss of enteric dopaminergic neurons and associated changes in colon motility in an MPTP mouse model of Parkinson’s disease. Exp Neurol. 2007;207:4–12.
Marrinan S, Emmanuel AV, Burn DJ. Delayed gastric emptying in Parkinson’s disease. Mov Disord. 2014;29:23–32.
Fasano A, Visanji NP, Liu LWC, Lang AE, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2015;14:625–639.
Kujawska M, Jodynis-Liebert J. What is the evidence that Parkinson’s disease is a prion disorder, which originates in the gut? Int J Mol Sci. 2018;19:3573.
Holmqvist S, Chutna O, Bousset L, et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128:805–820.
Ellett LJ, Hung LW, Munckton R, et al. Restoration of intestinal function in an MPTP model of Parkinson’s Disease. Sci Rep. 2016;6:30269.
Hilton D, Stephens M, Kirk L, et al. Accumulation of α-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta Neuropathol. 2014;127:235–241.
Shannon KM, Keshavarzian A, Mutlu E, et al. Alpha-synuclein in colonic submucosa in early untreated Parkinson’s disease. Mov Disord. 2012;27:709–715.
Stokholm MG, Danielsen EH, Hamilton-Dutoit SJ, Borghammer P. Pathological α-synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Ann Neurol. 2016;79:940–949.
Vittal H, Farrugia G, Gomez G, Pasricha PJ. Mechanisms of disease: the pathological basis of gastroparesis–a review of experimental and clinical studies. Nat Clin Pract Gastroenterol Hepatol. 2007;4:336–346.
Mukhopadhyay S, Sekhar KR, Hale AB, et al. Loss of NRF2 impairs gastric nitrergic stimulation and function. Free Radic Biol Med. 2011;51:619–625.
Gangula PRR, Mukhopadhyay S, Ravella K, et al. Tetrahydrobiopterin (BH4), a cofactor for nNOS, restores gastric emptying and nNOS expression in female diabetic rats. Am J Physiol Liver Physiol. 2010;298:G692–G699.
Gangula PRR, Mukhopadhyay S, Pasricha PJ, Ravella K. Sepiapterin reverses the changes in gastric nNOS dimerization and function in diabetic gastroparesis. Neurogastroenterol Motil. 2010;22:e351–e352.
Puspita L, Chung SY, Shim J-W. Oxidative stress and cellular pathologies in Parkinson’s disease. Mol Brain. 2017;10:53.
Cuanalo-Contreras K, Mukherjee A, Soto C. Role of protein misfolding and proteostasis deficiency in protein misfolding diseases and aging. Int J Cell Biol. 2013;2013:1–10.
Sweeney P, Park H, Baumann M, et al. Protein misfolding in neurodegenerative diseases: implications and strategies. Transl Neurodegener. 2017;6:6.
Hayes JD, McMahon M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci. 2009;34:176–188.
Lastres-Becker I, Ulusoy A, Innamorato NG, et al. α-Synuclein expression and Nrf2 deficiency cooperate to aggravate protein aggregation, neuronal death and inflammation in early-stage Parkinson’s disease. Hum Mol Genet. 2012;21:3173–3192.
Ishii T, Itoh K, Takahashi S, et al. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem. 2000;275:16023–16029.
Tufekci KU, Civi Bayin E, Genc S, Genc K. The Nrf2/ARE pathway: a promising target to counteract mitochondrial dysfunction in Parkinson’s disease. Parkinsons Dis. 2011;2011:1–14.
Hu R, Xu C, Shen G, et al. Gene expression profiles induced by cancer chemopreventive isothiocyanate sulforaphane in the liver of C57BL/6 J mice and C57BL/6 J/Nrf2 (−/−) mice. Cancer Lett. 2006;243:170–192.
Kwak M-K, Wakabayashi N, Greenlaw JL, Yamamoto M, Kensler TW. Antioxidants enhance mammalian proteasome expression through the Keap1-Nrf2 signaling pathway. Mol Cell Biol. 2003;23:8786–8794.
Skibinski G, Hwang V, Ando DM, et al. Nrf2 mitigates LRRK2- and α-synuclein-induced neurodegeneration by modulating proteostasis. Proc Natl Acad Sci USA. 2017;114:1165–1170.
Muthukumaran K, Smith J, Jasra H, et al. Genetic susceptibility model of Parkinson’s disease resulting from exposure of DJ-1 deficient mice to MPTP: evaluation of neuroprotection by Ubisol-Q10. J Parkinsons Dis. 2014;4:523–530.
Langston JW. The MPTP Story. J Parkinsons Dis. 2017;7:S11–S19.
Schmidt N, Ferger B. Neurochemical findings in the MPTP model of Parkinson’s disease. J Neural Transm. 2001;108:1263–1282.
Lotharius J, O’Malley KL. The Parkinsonism-inducing drug 1-Methyl-4-phenylpyridinium triggers intracellular dopamine oxidation. J Biol Chem. 2000;275:38581–38588.
King JM, Muthian G, Mackey V, Smith M, Charlton C. L-Dihydroxyphenylalanine modulates the steady-state expression of mouse striatal tyrosine hydroxylase, aromatic L-amino acid decarboxylase, dopamine and its metabolites in an MPTP mouse model of Parkinson’s disease. Life Sci. 2011;89:638–643.
Gangula PR, Chinnathambi V, Hale AB, Mukhopadhyay S, Channon KM, Ravella K. Impairment of nitrergic system and delayed gastric emptying in low density lipoprotein receptor deficient female mice. Neurogastroenterol Motil. 2011;23:773-e335.
Anitha M, Reichardt F, Tabatabavakili S, et al. Intestinal dysbiosis contributes to the delayed gastrointestinal transit in high-fat diet fed mice. Cell Mol Gastroenterol Hepatol. 2016;2:328–339.
Gangula PR, Challagundla KB, Ravella K, et al. Sepiapterin alleviates impaired gastric nNOS function in spontaneous diabetic female rodents through NRF2 mRNA turnover and miRNA biogenesis pathway. Am J Physiol Liver Physiol. 2018;315:G980–G990.
Gangula PRR, Sekhar KR, Mukhopadhyay S. Gender bias in gastroparesis: is nitric oxide the answer? Dig Dis Sci. 2011;56:2520–2527.
Ji C, Tang M, Johnson GVW. Assessing the degradation of tau in primary neurons: the role of autophagy. Methods Cell Biol. 2017;141:229–244.
Ali ZA, Rinze R, Douglas G, et al. Tetrahydrobiopterin determines vascular remodeling through enhanced endothelial cell survival and regeneration. Circulation. 2013;128:S50–S58.
Dahan D, Ekman M, Larsson-Callerfelt A-K, et al. Induction of angiotensin-converting enzyme after miR-143/145 deletion is critical for impaired smooth muscle contractility. Am J Physiol Physiol. 2014;307:C1093–C1101.
Greene JG, Noorian AR, Srinivasan S. Delayed gastric emptying and enteric nervous system dysfunction in the rotenone model of Parkinson’s disease. Exp Neurol. 2009;218:154–161.
Natale G, Kastsiushenka O, Fulceri F, Ruggieri S, Paparelli A, Fornai F. MPTP-induced parkinsonism extends to a subclass of TH-positive neurons in the gut. Brain Res. 2010;1355:195–206.
Sander LE, Lorentz A, Sellge G, et al. Selective expression of histamine receptors H1R, H2R, and H4R, but not H3R, in the human intestinal tract. Gut. 2006;55:498–504.
Hirst CS, Foong JPP, Stamp LA, et al. Ion channel expression in the developing enteric nervous system. PLoS One. 2015;10:e0123436.
Becker IL. Role of the transcription Factor Nrf2 in Parkinson’s disease: new insights. J Alzheimer’s Dis Park. 2017;07:340.
Williamson TP, Johnson DA, Johnson JA. Activation of the Nrf2-ARE pathway by siRNA knockdown of Keap1 reduces oxidative stress and provides partial protection from MPTP-mediated neurotoxicity. Neurotoxicology. 2012;33:272–279.
Chen P-C, Vargas MR, Pani AK, et al. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: critical role for the astrocyte. Proc Natl Acad Sci USA. 2009;106:2933–2938.
Gazaryan IG, Thomas B. The status of Nrf2-based therapeutics: current perspectives and future prospects. Neural Regen Res. 2016;11:1708–1711.
Johnson JA, Johnson DA, Kraft AD, et al. The Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Ann N Y Acad Sci. 2008;1147:61–69.
Cuadrado A, Manda G, Hassan A, et al. Transcription factor NRF2 as a therapeutic target for chronic diseases: a systems medicine approach. Pharmacol Rev. 2018;70:348–383.
Siebert A, Desai V, Chandrasekaran K, Fiskum G, Jafri MS. Nrf2 activators provide neuroprotection against 6-hydroxydopamine toxicity in rat organotypic nigrostriatal cocultures. J Neurosci Res. 2009;87:1659–1669.
Ebrahimi-Fakhari D, Cantuti-Castelvetri I, Fan Z, et al. Distinct roles in vivo for the ubiquitin-proteasome system and the autophagy-lysosomal pathway in the degradation of -synuclein. J Neurosci. 2011;31:14508–14520.
Todorovic M, Wood SA, Mellick GD. Nrf2: a modulator of Parkinson’s disease? J Neural Transm. 2016;123:611–619.
Sampath C, Sprouse JC, Freeman ML, Gangula PR. Activation of Nrf2 attenuates delayed gastric emptying in obesity induced diabetic (T2DM) female mice. Free Radic Biol Med. 2019;135:132–143.
Golpich M, Amini E, Hemmati F, et al. Glycogen synthase kinase-3 beta (GSK-3β) signaling: implications for Parkinson’s disease. Pharmacol Res. 2015;97:16–26.
Cuadrado A, Kügler S, Lastres-Becker I. Pharmacological targeting of GSK-3 and NRF2 provides neuroprotection in a preclinical model of tauopathy. Redox Biol. 2018;14:522–534.
David JA, Rifkin WJ, Rabbani PS, Ceradini DJ. The Nrf2/Keap1/ARE pathway and oxidative stress as a therapeutic target in type II diabetes mellitus. J Diabetes Res. 2017;2017:1–15.
Chandrasekharan B, Srinivasan S. Diabetes and the enteric nervous system. Neurogastroenterol Motil. 2007;19:951–960.
Paul EJ, Kalk E, Tossell K et al.: nNOS-expressing neurons in the ventral tegmental area and substantia nigra pars compacta. eneuro 2018;5:ENEURO.0381-18.2018.
Czarnecka A, Lenda T, Domin H, Konieczny J, Smiałowska M, Lorenc-Koci E. Alterations in the expression of nNOS in the substantia nigra and subthalamic nucleus of 6-OHDA-lesioned rats: the effects of chronic treatment with l-DOPA and the nitric oxide donor, molsidomine. Brain Res. 2013;1541:92–105.
Anitha M, Shahnavaz N, Qayed E, et al. BMP2 promotes differentiation of nitrergic and catecholaminergic enteric neurons through a Smad1-dependent pathway. Am J Physiol Gastrointest Liver Physiol. 2010;298:G375–G383.
Kim WS, Kågedal K, Halliday GM. Alpha-synuclein biology in Lewy body diseases. Alzheimers Res Ther. 2014;6:73.
López JM, Lozano D, Morales L, González A. Pattern of nitrergic neuronal system organization in the brain of two holostean fishes (Actinopterygii: Ginglymodi). Brain Behav Evol. 2017;89:117–152.
Pall ML. Nitric oxide synthase partial uncoupling as a key switching mechanism for the NO/ONOO- cycle. Med Hypotheses. 2007;69:821–825.
Kuiper MA, Teerlink T, Visser JJ, Bergmans PLM, Scheltens P, Wolters EC. L-Glutamate, l-arginine and L-citrulline levels in cerebrospinal fluid of Parkinson’s disease, multiple system atrophy, and Alzheimer’s disease patients. J Neural Transm. 2000;107:183–189.
Foxton RH, Land JM, Heales SJR. Tetrahydrobiopterin availability in Parkinson’s and Alzheimer’s disease; potential pathogenic mechanisms. Neurochem Res. 2007;32:751–756.
Crabtree MJ, Hale AB, Channon KM. Dihydrofolate reductase protects endothelial nitric oxide synthase from uncoupling in tetrahydrobiopterin deficiency. Free Radic Biol Med. 2011;50:1639–1646.
de Paula Martins R, Glaser V, Aguiar AS Jr, et al. De novo tetrahydrobiopterin biosynthesis is impaired in the inflammed striatum of parkin (−/−) mice. Cell Biol Int. 2018;42:725–733.
Ryan BJ, Lourenço-Venda LL, Crabtree MJ, Hale AB, Channon KM, Wade-Martins R. α-Synuclein and mitochondrial bioenergetics regulate tetrahydrobiopterin levels in a human dopaminergic model of Parkinson disease. Free Radic Biol Med. 2014;67:58–68.
Hölscher C. New drug treatments show neuroprotective effects in Alzheimer′s and Parkinson′s diseases. Neural Regen Res. 2014;9:1870.
Lee K-S, Lee J-K, Kim H-G, Kim HR. Differential effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine on motor behavior and dopamine levels at brain regions in three different mouse strains. Korean J Physiol Pharmacol. 2013;17:89.
Xue J, Yu C, Sheng W, et al. The Nrf2/GCH1/BH4 axis ameliorates radiation-induced skin injury by modulating the ROS cascade. J Invest Dermatol. 2017;137:2059–2068.
Takahashi S, Lin H, Geshi N, et al. Nitric oxide-cGMP-protein kinase G pathway negatively regulates vascular transient receptor potential channel TRPC6. J Physiol. 2008;586:4209–4223.
West AR, Tseng KY. Nitric oxide–soluble guanylyl cyclase–cyclic GMP signaling in the striatum: new targets for the treatment of parkinson’s disease? Front Syst Neurosci. 2011. https://doi.org/10.3389/fnsys.2011.00055.
Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2003;2:107–116.
Shang T, Kotamraju S, Zhao H, Kalivendi SV, Hillard CJ, Kalyanaraman B. Sepiapterin attenuates 1-methyl-4-phenylpyridinium-induced apoptosis in neuroblastoma cells transfected with neuronal NOS: role of tetrahydrobiopterin, nitric oxide, and proteasome activation. Free Radic Biol Med. 2005;39:1059–1074.
Lastres-Becker I, García-Yagüe AJ, Scannevin RH, et al. Repurposing the NRF2 activator dimethyl fumarate as therapy against synucleinopathy in Parkinson’s Disease. Antioxid Redox Signal. 2016;25:61–77.
Acknowledgment
The National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health (NIH) under Award Number SC1GM121282 supported research reported in this publication. Finally, we thank the Meharry Office for Scientific Editing and Publications for editorial support.
Author information
Authors and Affiliations
Contributions
P.G. contributed to conceptualization, methodology, and supervision; P. G. and K. R. were involved in data curation; C.S. and P. G. were involved in writing—original draft preparation; P. G., A. T., S. S., C. C., and C. K. M. were involved in writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sampath, C., Kalpana, R., Ansah, T. et al. Impairment of Nrf2- and Nitrergic-Mediated Gastrointestinal Motility in an MPTP Mouse Model of Parkinson’s Disease. Dig Dis Sci 64, 3502–3517 (2019). https://doi.org/10.1007/s10620-019-05693-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05693-5