Abstract
Background
α-Hederin has been shown promising anti-tumor potential against various cancer cell lines. However, reports about effects of α-hederin on esophageal squamous cell carcinoma (ESCC) are still unavailable.
Aim
To investigate the inhibitory effects of α-hederin on ESCC and explore the underlying mechanism.
Methods
Human esophageal carcinoma cell line (Eca-109) was used for the experiment. Cell Counting Kit-8, flow cytometry, Hoechst 33258 staining, enhanced ATP assay kit, 2′,7′-dichlorofluorescin diacetate, JC-1 kit, and Western bolt were used to assess the cell viability, cycle, apoptosis, cellular ATP content, reactive oxygen species (ROS) level, mitochondrial membrane potential (MMP), and protein expression, respectively, in vitro. Xenografted tumor model was constructed to evaluate the in vivo anti-tumor effects of α-hederin.
Results
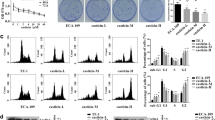
Compared with control group, α-hederin significantly inhibited the proliferation, induced apoptosis of ESCC, and arrested the cell cycle in G1 phase (P < 0.05). α-Hederin induced the accumulation of ROS, decrement of ATP levels, and disruption of MMP (P < 0.05). The detection of mitochondrial and cytosol proteins showed that AIF, Apaf-1, and Cyt C were released and increased in cytoplasm, and then, caspase-3, caspase-9, and Bax were involved and increased, while Bcl-2 level was decreased (P < 0.05). Furthermore, the above changes were amplified in the group pretreated with l-buthionine sulfoximine, while N-acetyl-l-cysteine plays an opposite role (P < 0.05). Meanwhile, α-hederin significantly inhibited the growth of xenografted tumors with favorable safety.
Conclusion
α-Hederin could inhibit the proliferation and induce apoptosis of ESCC via dissipation of the MMP with simultaneous ROS generation and activation of the mitochondrial pathway.







Similar content being viewed by others
References
Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA A Cancer J Clin. 2015;65:87–108.
Abnet CC, Arnold M, Wei WQ. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology. 2018;154:360–373.
Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet. 2017;390:2383–2396.
Haas SL, Ye W, Lohr JM. Alcohol consumption and digestive tract cancer. Curr Opin Clin Nutr Metab Care. 2012;15:457–467.
Chen Y, Tong Y, Yang C, et al. Consumption of hot beverages and foods and the risk of esophageal cancer: a meta-analysis of observational studies. BMC Cancer. 2015;15:449.
Matejcic M, Iqbal PM. Gene-environment interactions in esophageal cancer. Crit Rev Clin Lab Sci. 2015;52:211–231.
Sawada G, Niida A, Uchi R, et al. Genomic landscape of esophageal squamous cell carcinoma in a Japanese population. Gastroenterology. 2016;150:1171–1182.
Liu J, Wang J, Leng Y, Lv C. Intake of fruit and vegetables and risk of esophageal squamous cell carcinoma: a meta-analysis of observational studies. Int J Cancer. 2013;133:473–485.
Yamashita K, Katada N, Moriya H, et al. Multimodality treatment and prognosis in esophageal squamous cell carcinoma requiring esophagectomy. Hepatogastroenterology. 2014;61:1042–1048.
Kadota T, Hatogai K, Yano T, et al. Pathological tumor regression grade of metastatic tumors in lymph node predicts prognosis in esophageal cancer patients. Cancer Sci. 2018;109:2046–2055.
Njei B, McCarty TR, Birk JW. Trends in esophageal cancer survival in United States adults from 1973 to 2009: a SEER database analysis. J Gastroenterol Hepatol. 2016;31:1141–1146.
Li J, Gong Y, Diao P, et al. Comparison of the clinical efficacy between single-agent and dual-agent concurrent chemoradiotherapy in the treatment of unresectable esophageal squamous cell carcinoma: a multicenter retrospective analysis. Radiat Oncol. 2018;13:12.
Rooney S, Ryan MF. Effects of α-hederin and thymoquinone, constituents of Nigella sativa, on human cancer cell lines. Anticancer Res. 2005;25:2199–2204.
Lorent JH, Leonard C, Abouzi M, et al. α-Hederin induces apoptosis, membrane permeabilization and morphologic changes in two cancer cell lines through a cholesterol-dependent mechanism. Planta Med. 2016;82:1532–1539.
Fallahi M, Keyhanmanesh R, Khamaneh AM, et al. Effect of α-hederin, the active constituent of Nigella sativa, on miRNA-126, IL-13 mRNA levels and inflammation of lungs in ovalbumin-sensitized male rats. Avic J Phytomed. 2016;6:77–85.
Keyhanmanesh R, Saadat S, Mohammadi M, Shahbazfar AA, Fallahi M. The protective effect of α-hederin, the active constituent of nigella sativa, on lung inflammation and blood cytokines in ovalbumin sensitized guinea pigs. Phytother Res. 2015;29:1761–1767.
Gepdiremen A, Mshvildadze V, Suleyman H, Elias R. Acute anti-inflammatory activity of four saponins isolated from ivy: α-hederin, hederasaponin-C, hederacolchiside-E and hederacolchiside-F in carrageenan-induced rat paw edema. Phytomedicne. 2005;12:440–444.
Li J, Wu DD, Zhang JX, et al. Mitochondrial pathway mediated by reactive oxygen species involvement in α-hederin-induced apoptosis in hepatocellular carcinoma cells. World J Gastroenterol. 2018;24:1901–1910.
Cheng L, Xia TS, Wang YF, et al. The anticancer effect and mechanism of α-hederin on breast cancer cells. Int J Oncol. 2014;45:757–763.
Sodrul I, Wang C, Chen X, Du J, Sun H. Role of ginsenosides in reactive oxygen species-mediated anticancer therapy. Oncotarget. 2018;9:2931–2950.
Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Semin Cell Dev Biol. 2018;80:50–64.
Sosa V, Moline T, Somoza R, et al. Oxidative stress and cancer: an overview. Ageing Res Rev. 2013;12:376–390.
Wu WS. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006;25:695–705.
Weinberg F, Chandel NS. Reactive oxygen species-dependent signaling regulates cancer. Cell Mol Life Sci. 2009;66:3663–3673.
Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat Rev Cancer. 2014;14:709–721.
Ding Y, Wang H, Niu J, et al. Induction of ROS overload by alantolactone prompts oxidative DNA damage and apoptosis in colorectal cancer cells. Int J Mol Sci. 2016;17:558.
Aredia F, Czaplinski S, Fulda S, Scovassi AI. Molecular features of the cytotoxicity of an NHE inhibitor: evidence of mitochondrial alterations, ROS overproduction and DNA damage. BMC Cancer. 2016;16:851.
Aquilano K, Baldelli S, Ciriolo MR. Glutathione: new roles in redox signaling for an old antioxidant. Front Pharmacol. 2014;5:196.
Yan MH, Wang X, Zhu X. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic Biol Med. 2013;62:90–101.
Bertero E, Maack C. Calcium signaling and reactive oxygen species in mitochondria. Circ Res. 2018;122:1460–1478.
Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136.
Reubold TF, Wohlgemuth S, Eschenburg S. A new model for the transition of APAF-1 from inactive monomer to caspase-activating apoptosome. J Biol Chem. 2009;284:32717–32724.
Zhou M, Li Y, Hu Q, et al. Atomic structure of the apoptosome: mechanism of cytochrome c- and dATP-mediated activation of Apaf-1. Genes Dev. 2015;29:2349–2361.
Edlich F. BCL-2 proteins and apoptosis: recent insights and unknowns. Biochem Biophys Res Commun. 2018;500:26–34.
Kelekar A, Thompson CB. Bcl-2-family proteins: the role of the BH3 domain in apoptosis. Trends Cell Biol. 1998;8:324–330.
Acknowledgments
This study is supported by the National Natural Science Foundation of China (No. 81572426), the Natural Science Foundation of Hubei Province (No. 2015CKB755) and the Youth Fund of Beijing Shijitan Hospital (No. 2016-q07).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, J., Wu, D., Zhang, J. et al. α-Hederin Induces Apoptosis of Esophageal Squamous Cell Carcinoma via an Oxidative and Mitochondrial-Dependent Pathway. Dig Dis Sci 64, 3528–3538 (2019). https://doi.org/10.1007/s10620-019-05689-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05689-1