Abstract
Background
Liver fibrosis can progress to cirrhosis, hepatocellular carcinoma, or liver failure. Unfortunately, the antifibrotic agents are limited. Thrombin activates hepatic stellate cells (HSCs). Therefore, we investigated the effects of a direct thrombin inhibitor, dabigatran, on liver fibrosis.
Methods
Adult male Sprague–Dawley rats were injected intraperitoneally with thioacetamide (TAA, 200 mg/kg twice per week) for 8 or 12 weeks to induce liver fibrosis. The injured rats were assigned an oral gavage of dabigatran etexilate (30 mg/kg/day) or vehicle in the last 4 weeks of TAA administration. Rats receiving an injection of normal saline and subsequent oral gavage of dabigatran etexilate or vehicle served as controls.
Results
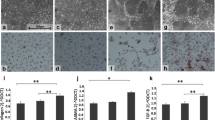
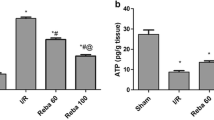
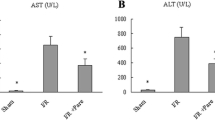
In the 8-week TAA-injured rats, dabigatran ameliorated fibrosis, fibrin deposition, and phosphorylated ERK1/2 in liver, without altering the transcript expression of thrombin receptor protease-activated receptor-1. In vitro, dabigatran inhibited thrombin-induced HSC activation. Furthermore, dabigatran reduced intrahepatic angiogenesis and portal hypertension in TAA-injured rats. Similarly, in the 12-week TAA-injured rats, a 4-week treatment with dabigatran reduced liver fibrosis and portal hypertension.
Conclusions
By inhibiting thrombin action, dabigatran reduced liver fibrosis and intrahepatic angiogenesis. Dabigatran may be a promising therapeutic agent for treatment of liver fibrosis.






Similar content being viewed by others
References
Friedman SL. Liver fibrosis—from bench to bedside. J Hepatol. 2003;38:S38–S53.
Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669.
Pinzani M, Milani S, De Franco R, et al. Endothelin 1 is overexpressed in human cirrhotic liver and exerts multiple effects on activated hepatic stellate cells. Gastroenterology. 1996;110:534–548.
Popov Y, Schuppan D. Targeting liver fibrosis: strategies for development and validation of antifibrotic therapies. Hepatology. 2009;50:1294–1306.
Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264.
Sullivan BP, Weinreb PH, Violette SM, Luyendyk JP. The coagulation system contributes to alphaVbeta6 integrin expression and liver fibrosis induced by cholestasis. Am J Pathol. 2010;177:2837–2849.
Fiorucci S, Antonelli E, Distrutti E, et al. PAR1 antagonism protects against experimental liver fibrosis. Role of proteinase receptors in stellate cell activation. Hepatology. 2004;39:365–375.
Rhea JM, Molinaro RJ. Direct thrombin inhibitors: clinical uses, mechanism of action, and laboratory measurement. MLO Med Lab Obs. 2011;43:20–22.
Kopec AK, Joshi N, Towery KL, et al. Thrombin inhibition with dabigatran protects against high-fat diet-induced fatty liver disease in mice. J Pharmacol Exp Ther. 2014;351:288–297.
Kassel KM, Sullivan BP, Cui W, Copple BL, Luyendyk JP. Therapeutic administration of the direct thrombin inhibitor argatroban reduces hepatic inflammation in mice with established fatty liver disease. Am J Pathol. 2012;181:1287–1295.
Eisert WG, Hauel N, Stangier J, Wienen W, Clemens A, van Ryn J. Dabigatran: an oral novel potent reversible nonpeptide inhibitor of thrombin. Arterioscler Thromb Vasc Biol. 2010;30:1885–1889.
van Ryn J, Schurer J, Kink-Eiband M, Clemens A. Reversal of dabigatran-induced bleeding by coagulation factor concentrates in a rat-tail bleeding model and lack of effect on assays of coagulation. Anesthesiology. 2014;120:1429–1440.
Lee KC, Yang YY, Huang YT, et al. Administration of a low dose of sildenafil for 1 week decreases intrahepatic resistance in rats with biliary cirrhosis: the role of NO bioavailability. Clin Sci. 1979;119:45–55.
Cerini F, Vilaseca M, Lafoz E, et al. Enoxaparin reduces hepatic vascular resistance and portal pressure in cirrhotic rats. J Hepatol. 2016;64:834–842.
Blackburn JS, Brinckerhoff CE. Matrix metalloproteinase-1 and thrombin differentially activate gene expression in endothelial cells via PAR-1 and promote angiogenesis. Am J Pathol. 2008;173:1736–1746.
Fernandez M, Semela D, Bruix J, Colle I, Pinzani M, Bosch J. Angiogenesis in liver disease. J Hepatol. 2009;50:604–620.
Gao JH, Wen SL, Yang WJ, et al. Celecoxib ameliorates portal hypertension of the cirrhotic rats through the dual inhibitory effects on the intrahepatic fibrosis and angiogenesis. PloS One. 2013;8:e69309.
Gao JH, Wen SL, Feng S, et al. Celecoxib and octreotide synergistically ameliorate portal hypertension via inhibition of angiogenesis in cirrhotic rats. Angiogenesis. 2016;19:501–511.
Zhang J, Luo B, Tang L, et al. Pulmonary angiogenesis in a rat model of hepatopulmonary syndrome. Gastroenterology. 2009;136:1070–1080.
Wanless IR, Wong F, Blendis LM, Greig P, Heathcote EJ, Levy G. Hepatic and portal vein thrombosis in cirrhosis: possible role in development of parenchymal extinction and portal hypertension. Hepatology. 1995;21:1238–1247.
Violi F, Ferro D, Basili S, et al. Increased rate of thrombin generation in hepatitis C virus cirrhotic patients. Relationship to venous thrombosis. J Investig Med. 1995;43:550–554.
Marra F, Grandaliano G, Valente AJ, Abboud HE. Thrombin stimulates proliferation of liver fat-storing cells and expression of monocyte chemotactic protein-1: potential role in liver injury. Hepatology. 1995;22:780–787.
Knight V, Tchongue J, Lourensz D, Tipping P, Sievert W. Protease-activated receptor 2 promotes experimental liver fibrosis in mice and activates human hepatic stellate cells. Hepatology. 2012;55:879–887.
Kukla M. Angiogenesis: a phenomenon which aggravates chronic liver disease progression. Hepatol Int. 2013;7:4–12.
Novo E, Cannito S, Zamara E, et al. Proangiogenic cytokines as hypoxia-dependent factors stimulating migration of human hepatic stellate cells. Am J Pathol. 2007;170:1942–1953.
Mochizuki A, Pace A, Rockwell CE, et al. Hepatic stellate cells orchestrate clearance of necrotic cells in a hypoxia-inducible factor-1alpha-dependent manner by modulating macrophage phenotype in mice. J Immunol. 2014;192:3847–3857.
Zhao Y, Wang Y, Wang Q, Liu Z, Liu Q, Deng X. Hepatic stellate cells produce vascular endothelial growth factor via phospho-p44/42 mitogen-activated protein kinase/cyclooxygenase-2 pathway. Mol Cell Biochem. 2012;359:217–223.
Wang Y, Huang Y, Guan F, et al. Hypoxia-inducible factor-1alpha and MAPK co-regulate activation of hepatic stellate cells upon hypoxia stimulation. PloS One. 2013;8:e74051.
Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365:147–156.
Sogaard KK, Horvath-Puho E, Gronbaek H, Jepsen P, Vilstrup H, Sorensen HT. Risk of venous thromboembolism in patients with liver disease: a nationwide population-based case-control study. Am J Gastroenterol. 2009;104:96–101.
Tripodi A, Fracanzani AL, Primignani M, et al. Procoagulant imbalance in patients with nonalcoholic fatty liver disease. J Hepatol. 2014;61:148–154.
Villa E, Camma C, Marietta M, et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology. 2012;143:e1251–e1254.
Vilaseca M, Garcia-Caldero H, Lafoz E, et al. The anticoagulant rivaroxaban lowers portal hypertension in cirrhotic rats mainly by deactivating hepatic stellate cells. Hepatology. 2017;65:2031–2044.
De Gottardi A, Trebicka J, Klinger C, et al. Antithrombotic treatment with direct-acting oral anticoagulants in patients with splanchnic vein thrombosis and cirrhosis. Liver Int. 2017;37:694–699.
Graff J, Harder S. Anticoagulant therapy with the oral direct factor Xa inhibitors rivaroxaban, apixaban and edoxaban and the thrombin inhibitor dabigatran etexilate in patients with hepatic impairment. Clin Pharmacokinet. 2013;52:243–254.
Ebner T, Wagner K, Wienen W. Dabigatran acylglucuronide, the major human metabolite of dabigatran: in vitro formation, stability, and pharmacological activity. Drug Metab Dispos. 2010;38:1567–1575.
Iwakiri Y, Shah V, Rockey DC. Vascular pathobiology in chronic liver disease and cirrhosis: current status and future directions. J Hepatol. 2014;61:912–924.
Bosch J, Berzigotti A, Garcia-Pagan JC, Abraldes JG. The management of portal hypertension: rational basis, available treatments and future options. J Hepatol. 2008;48:S68–S92.
Acknowledgments
The authors gratefully acknowledge Miss Chia-Li Chen for her technical assistance.
Funding
Research reported in this publication was supported by the Ministry of Science and Technology, Taiwan (NSC.104-2314-B-075-023-MY2), and the Taipei Veterans General Hospital (V103C-012).
Author information
Authors and Affiliations
Contributions
KC Lee, WF Hsu, and HC Lin conceived and designed the study. KC Lee, WF Hsu, YC Hsieh, and YY Yang analyzed and interpreted data. KC Lee and WF Hsu drafted the manuscript. KC Lee, WF Hsu, YH Huang, and HC Lin critically revised the article for important intellectual content. KC Lee, WF Hsu, YC Hsieh, YY Yang, YH Huang, and HC Lin approved the final approval of the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, KC., Hsu, WF., Hsieh, YC. et al. Dabigatran Reduces Liver Fibrosis in Thioacetamide-Injured Rats. Dig Dis Sci 64, 102–112 (2019). https://doi.org/10.1007/s10620-018-5311-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-018-5311-1