Abstract
Background
Cell division cycle 42 (CDC42), an important member of the Rho family, is overexpressed in various human cancers. However, its expression and role in pancreatic cancer (PC) are not well understood.
Aim
The present study was designed to investigate the expression patterns and underlying cellular mechanisms of CDC42 in PC.
Methods
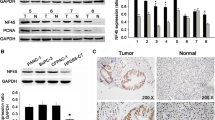
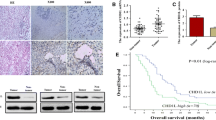
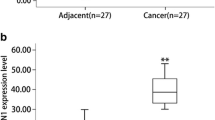
First, immunohistochemical analysis, quantitative real-time polymerase chain reaction (qRT-PCR) and Western blotting were performed to detect CDC42 expression in clinical pancreatic carcinoma and adjacent tissues. Second, differential expression of CDC42 between PC cells and normal cells was evaluated by qRT-PCR and Western blotting. Third, the correlation between CDC42 expression as well as clinicopathological characteristics and patient survival was analyzed. Finally, CDC42 was knocked down to examine its role both in vivo and in vitro.
Results
The results showed significantly increased CDC42 expression in pancreatic tumor tissues compared with adjacent normal tissues, as revealed by qRT-PCR, Western blotting and immunostaining. Compared to PanC-1 cells, CDC42 expression was downregulated in HPDE6-C7 cells as shown by qRT-PCR and Western blotting. High CDC42 expression was observed in 69.2% (83/120) of pancreatic adenocarcinoma patients and was significantly associated with tumor differentiation (p = 0.013), median tumor size (p = 0.005), tumor infiltration (pT stage, p = 0.04), lymph nodal status (pN stage, p = 0.044) and TNM staging (p = 0.003). Multivariate Cox regression analysis revealed CDC42 expression to be an independent predictor of survival of PC patients (HR 3.0, 95% CI 1.60–5.61, p = 0.001). Finally, we found that CDC42 promoted the proliferation of PanC-1 cells both in vivo and in vitro.
Conclusions
Our findings reveal that CDC42 might play an important role in promoting PC development, and the findings suggest that CDC42 might serve as a potential prognostic indicator of PC.



Similar content being viewed by others
References
Douglas H, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674.
Siegel RebeccaL, Miller KimberlyD, Jemal Ahmedin. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29.
He Jin, Page AJ, Weiss M, Wolfgang CL, Herman JM, Pawlik TM. Management of borderline and locally advanced pancreatic cancer: Where do we stand? World J Gastroenterol. 2014;20:2255–2266.
Ke Kun, Chen Wen, Chen Yanling. Standard and extended lymphadenectomy for adenocarcinoma of the pancreatic head: a meta-analysis and systematic review. J Gastroenterol Hepatol. 2014;29:453–462.
Ji M, Yan X, Wenchao L, et al. Role of activated Rac1/Cdc42 in mediating endothelial cell proliferation and tumor angiogenesis in breast cancer. PLoS ONE. 2013;8:e66275.
Zhang JY, Di Z, Wang EH. Overexpression of small GTPases directly correlates with expression of δ-catenin and their coexpression predicts a poor clinical outcome in nonsmall cell lung cancer. Mol Carcinog. 2013;52:338–347.
Fritz G, Brachetti C, Bahlmann F, Schmidt M, Kaina B. Rho GTPases in human breast tumours: expression and mutation analyses and correlation with clinical parameters. Br J Cancer. 2002;87:635–644.
Gómez DPT, Valdés-Mora F, Bandrés E, et al. Cdc42 is highly expressed in colorectal adenocarcinoma and downregulates ID4 through an epigenetic mechanism. Int J Oncol. 2008;33:185–193.
Abraham MT, Abraham Kuriakose M, Sacks PG, et al. Motility-related proteins as markers for head and neck squamous cell cancer. Laryngoscope. 2001;11:1285–1289.
Zuo Y, Wu Y, Wehrli B, Chakrabarti S, Chakraborty C. Modulation of ERK5 is a novel mechanism by which Cdc42 regulates migration of breast cancer cells. J Cell Biochem. 2015;116:124–132.
Cerqueira OL, Truesdell P, Baldassarre T, et al. Cip4 promotes metastasis in triple-negative breast cancer and is associated with poor patient prognosis. Oncotarget. 2015;6:9397–9408.
Lee S, Craig BT, Romain CV, Qiao J, Dai HC. Silencing of CDC42 inhibits neuroblastoma cell proliferation and transformation. Cancer Lett. 2014;355:210–216.
Tucci MG, Lucarini G, Brancorsini D, et al. Involvement of E-cadherin, β-catenin, Cdc42 and CXCR4 in the progression and prognosis of cutaneous melanoma. Br J Dermatol. 2007;157:1212–1216.
Cai S, Ye Z, Wang X, et al. Overexpression of p21-activated kinase 4 is associated with poor prognosis in non-small cell lung cancer and promotes migration and invasion. J Exp Clin Cancer Res. 2015;34:1–10.
Tseng RC, Chang JM, Chen JH, et al. Deregulation of slit2-mediated cdc42 activity is associated with esophageal cancer metastasis and poor prognosis. J Thorac Oncol. 2014;10:189–198.
Wang B, Xu T, Liu J. Overexpression of activated cdc42-associated kinase1 (ack1) predicts tumor recurrence and poor survival in human hepatocellular carcinoma. Pathol Res Pract. 2014;210:787–792.
Liu M, Lang N, Qiu M, et al. miR-137 targets Cdc42 expression, induces cell cycle G1 arrest and inhibits invasion in colorectal cancer cells. Int J Cancer. 2011;128:1269–1279.
Yang D, Zhu Z, Wang W, et al. Expression profiles analysis of pancreatic cancer. Eur Rev Med Pharmacol Sci. 2013;17:311–317.
Cheng YJ, Zhu ZX, Zhou JS, et al. Silencing profilin-1 inhibits gastric cancer progression via integrin β1/focal adhesion kinase pathway modulation. World J Gastroenterol. 2015;21:2323–2335.
Sun X, Qiu JJ, Zhu S, et al. Oncogenic features of PHF8 histone demethylase in esophageal squamous cell carcinoma. PLoS ONE. 2013;8:e77353.
Hidalgo M, Cascinu S, Kleeff J, et al. Addressing the challenges of pancreatic cancer: future directions for improving outcomes. Pancreatology. 2015;15:8–18.
Gómez del Pulgar T, Benitah SA, Valeron PF, Espina C, Lacal JC. Rho GTPase expression in tumourigenesis: evidence for a significant link. BioEssays. 2005;27:602–613.
Etienne-Manneville S. Cdc42—the centre of polarity. J Cell Sci. 2004;117:1291–1300.
Stengel K, Yi Z. Cdc42 in oncogenic transformation, invasion, and tumorigenesis. Cell Signal. 2011;23:1415–1423.
Dan J, Joanne D, Alan H. Functional cross-talk between Cdc42 and two downstream targets, Par6B and PAK4. Biochem J. 2015;467:293–302.
Hongnan Y, Youyi Z, Li G, Zijian L. Cdc42 expression in cervical cancer and its effects on cervical tumor invasion and migration. Int J Oncol. 2015;46:757–763.
Orgaz JL, Herraiz C, Sanz-Moreno V. Rho GTPases modulate malignant transformation of tumor cells. Small GTPases. 2014;5:e29019.
Satish M, Fasiha K, Hong X, Banke A. New diagnosis of chronic pancreatitis: risk of missing an underlying pancreatic cancer. Am J Gastroenterol. 2014;110:1824–1830.
Ito TK, Yokoyama M, Yoshida Y, et al. A crucial role for CDC42 in senescence-associated inflammation and atherosclerosis. PLoS ONE. 2014;9:e102186.
Dammann K, Khare V, Gasche C. Tracing PAKs from GI inflammation to cancer. Gut. 2014;63:1173–1184.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81372670 and 81402359) and Scientific Research Program of Shanghai Municipal Commission of Health and Family Planning (201640269 and 2014176). The authors are grateful to the Department of Biochemistry and Molecular Biology, Second Military Medical University, and to Prof. Wang Lianghua for their technical support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Dejun Yang, Yu Zhang and Yajun Cheng have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yang, D., Zhang, Y., Cheng, Y. et al. High Expression of Cell Division Cycle 42 Promotes Pancreatic Cancer Growth and Predicts Poor Outcome of Pancreatic Cancer Patients. Dig Dis Sci 62, 958–967 (2017). https://doi.org/10.1007/s10620-017-4451-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-017-4451-z