Abstract
Background/Aim
How to prevent the small intestinal damage induced by NSAIDs is an urgent issue to be resolved. In the present study, we examined the effects of soluble dietary fibers on both anti-inflammatory and ulcerogenic effects of indomethacin in arthritic rats.
Methods
Male Wistar rats weighing 180–220 g were used. Arthritis was induced by injecting Freund’s complete adjuvant (killed M. tuberculosis) into the plantar region of the right hindpaw. The animals were fed a regular powder diet for rats or a diet supplemented with soluble dietary fibers such as pectin or guar gum. Indomethacin was administered once a day for 3 days starting 14 days after the adjuvant injection, when marked arthritis was observed. The volumes of the hindpaw were measured before and after indomethacin treatment to evaluate the effect of indomethacin on edema. The lesions in the small intestine were examined 24 h after the final dosing of indomethacin.
Results
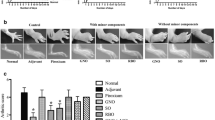
Hindpaw volume was increased about 3 times 14 days after injection of the adjuvant. Indomethacin (3–10 mg/kg, p.o.) decreased hindpaw volume dose-dependently, but caused severe lesions in the small intestine at doses of 6 and 10 mg/kg. The addition of pectin (1–10 %) or guar gum (10 %) to the diet markedly decreased the lesion formation without affecting the anti-edema action of indomethacin. The same effects of pectin were observed when indomethacin was administered subcutaneously.
Conclusions
It is suggested that soluble dietary fibers can prevent intestinal damage induced by NSAIDs without affecting the anti-inflammatory effect of these agents.







Similar content being viewed by others
References
Graham DY, Opekum AR, Willingham FF, et al. Visible small-intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol. 2005;3:55–59.
Maiden L, Thjodleifsson B, Theodors A, et al. A quantitative analysis of NSAID induced small bowel pathology by capsule enteroscopy. Gastroenterology. 2005;128:1172–1178.
Goldstein JL, Eisen GM, Lewis B, et al. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol. 2005;3:133–141.
Matsumoto T, Kudo T, Esaki M, et al. Prevalence of nonsteroidal anti-inflammatory drug-induced enteropathy determined by double-balloon endoscopy: a Japanese multicenter study. Scand J Gastroenterol. 2008;43:490–496.
Leung WK, Bjarnason I, Wong VW, et al. Small bowel enteropathy associated with chronic low-dose aspirin therapy. Lancet. 2007;369:614.
Shiotani A. Low-dose aspirin-induced gastrointestinal diseases: past, present, and future. J Gastroenterol. 2008;43:581–588.
Endo H, Hosono K, Inamori M, et al. Characteristics of small bowel injury in symptomatic chronic low-dose aspirin users: the experience of two medical centers in capsule endoscopy. J Gastroenterol. 2009;44:544–549.
Bjarnason I, Smethurst P, Fenn CG, et al. Misoprostol reduces indomethacin-induced changes in human small intestinal permeability. Dig Dis Sci. 1981;34:407–411.
Watanabe T, Sugimori S, Kameda N, et al. Small bowel injury by low-dose enteric-coated aspirin and treatment with misoprostol: a pilot study. Clin Gastroenterol Hepatol. 2008;6:1279–1282.
Fujimori S, Seo T, Gudis K, et al. Prevention of nonsteroidal anti-inflammatory drug-induced small-intestinal injury by prostaglandin: a pilot randomized controlled trial evaluated by capsule endoscopy. Gastrointest Endosc.. 2009;69:1339–1346.
Watanabe T, Nishio H, Tanigawa T, et al. Probiotic Lactbacillus casei strain Shirota prevents indomethacin-induced small intestinal injury: involvement of lactic acid. Am J Physiol Gastrointest Liver Physiol.. 2009;297:G506–G513.
Mizoguchi H, Ogawa Y, Kanatsu K, et al. Protective effect of rebamipide on indomethacin-induced intestinal damage in rats. J Gastroenterol Hepatol. 2001;16:1112–1119.
Kamei K, Kubo Y, Kato N, et al. Prophylactic effect of irsogladine maleate against indomethacin-induced small intestinal lesions in rats. Dig Dis Sci. 2008;53:2657–2666.
Satoh H, Amagase K, Takeuchi K. Mucosal protective agents prevent exacerbation of NSAID-induced small intestinal lesions caused by antisecretory drugs in rats. J Pharmacol Exp Ther. 2012;348:227–235.
Satoh H, Takeuchi K. Management of NSAID/aspirin-induced small intestinal damage by GI-sparing NSAIDs, anti-ulcer drugs and food constituents. Curr Med Chem. 2012;19:82–89.
Satoh H, Hara T, Murakawa D, et al. Soluble dietary fiber protects against nonsteroidal anti-inflammatory drug-induced damage to the small intestine in cats. Dig Dis Sci. 2010;55:1264–1271.
Satoh H, Amagase K, Takeuchi K. The role of food for the formation and prevention of gastrointestinal lesions induced by aspirin in cats. Dig Dis Sci. 2013;58:2840–2849.
Fries JF, Miller SR, Spitz PW, et al. (1989) Toward an epidemiology of gastropathy associated with nonsteroidal antiinflammatory drug use. Gastroenterology. 6:647-55.
Kato S, Tanaka A, Kunikata T, et al. Changes in gastric mucosal ulcerogenic responses in rats with adjuvant arthritis: role of nitric oxide. Aliment Pharmacol Ther. 1999;13:833–840.
Kato S, Ito Y, Nishio H, et al. Increased susceptibility of small intestine to NSAID-provoked ulceration in rats with adjuvant-induced arthritis: involvement of enhanced expression of TLR4. Life Sci. 2007;81:1309–1316.
Winter CA, Nuss GW. Treatment of adjuvant arthritis in rats with anti-inflammatory drugs. Arthritis Rheum. 1966;9:394–404.
Satoh H, Guth PH, Grossman MI. Role of food in gastrointestinal ulceration produced by indomethacin in the rat. Gastroenterology. 1982;83:210–215.
Satoh H, Shiotani S, Otsuka N, et al. Role of dietary fibres, intestinal hypermotility and leukotrienes in the pathogenesis of NSAID-induced small intestinal ulcers in cats. Gut. 2009;58:1590–1596.
Satoh H. Role of dietary fiber in formation and prevention of small intestinal ulcers induced by nonsteroidal anti-inflammatory drug. Curr Pharm Des. 2010;16:1209–1213.
Takeuchi K, Satoh H. NSAID-induced small intestinal damage—roles of various pathogenic factors. Digestion. 2015;91:218–232.
Acknowledgments
The authors are greatly indebted to Dr. Yasutada Akiba, Center for Ulcer Research and Education/University of California, Los Angeles Medical School, Los Angeles, CA, USA, for his valuable discussions and suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Satoh, H., Matsumoto, H., Hirakawa, T. et al. Soluble Dietary Fibers Can Protect the Small Intestinal Mucosa Without Affecting the Anti-inflammatory Effect of Indomethacin in Adjuvant-Induced Arthritis Rats. Dig Dis Sci 61, 91–98 (2016). https://doi.org/10.1007/s10620-015-3889-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-015-3889-0