Abstract
Background
VEGF-induced vascular permeability and blood vessels remodeling are key features of inflammatory bowel disease (IBD) pathogenesis. Dopamine through D2 receptor (D2R) inhibits VEGF/VPF-mediated vascular permeability and angiogenesis in tumor models. In this study, we tested the hypothesis that pathogenesis of IBD is characterized by the disturbance of dopaminergic system and D2R activity.
Methods
IL-10 knockout (KO) mice and rats with iodoacetamide-induced ulcerative colitis (UC) were treated intragastrically with D2R agonists quinpirole (1 mg/100 g) or cabergoline (1 or 5 µg/100 g). Macroscopic, histologic, and clinical features of IBD, colonic vascular permeability, and angiogenesis were examined.
Results
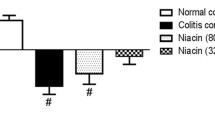
Although colonic D2R protein increased, levels of tyrosine hydroxylase and dopamine transporter DAT decreased in both models of IBD. Treatment with quinpirole decreased the size of colonic lesions in rats with iodoacetamide-induced UC (p < 0.01) and reduced colon wet weight in IL-10 KO mice (p < 0.05). Quinpirole decreased colonic vascular permeability (p < 0.001) via downregulation of c-Src and Akt phosphorylation. Cabergoline (5 µg/100 g) reduced vascular permeability but did not affect angiogenesis and improved signs of iodoacetamide-induced UC in rats (p < 0.05).
Conclusions
Treatment with D2R agonists decreased the severity of UC in two animal models, in part, by attenuation of enhanced vascular permeability and prevention of excessive vascular leakage. Hence, the impairment dopaminergic system seems to be a feature of IBD pathogenesis.






Similar content being viewed by others
References
Burisch J, Jess T, Martinato M, et al. The burden of inflammatory bowel disease in Europe. J Crohns Colitis. 2013;7:322–337.
Rocchi A, Benchimol EI, Bernstein CN, et al. Inflammatory bowel disease: a Canadian burden of illness review. Can J Gastroenterol. 2012;26:811–817.
Ording AG, Horváth-Puhó E, Erichsen R, et al. Five-year mortality in colorectal cancer patients with ulcerative colitis or Crohn’s disease: a nationwide population-based cohort study. Inflamm Bowel Dis. 2013;19:800–805.
Brahme F, Lindstrom C. A comparative radiographic and pathological study of intestinal vaso-architecture in Crohn’s disease and in ulcerative colitis. Gut. 1970;11:928–940.
Chidlow JH Jr, Langston W, Greer JJM, et al. Differential angiogenic regulation of experimental colitis. Am J Pathol. 2006;169:2014–2030.
Danese S, Sans M, Motte CDL, et al. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroeneterology. 2006;130:2060–2073.
Foitzik T, Kruschewski M, Kroesen A, et al. Does microcirculation play a role in the pathogenesis of inflammatory bowel diseases? Answers from intravital microscopic studies in animal models. Int J Colorectal Dis. 1999;14:29–34.
Hatoum OA, Miura H, Binion DG. The vascular contribution in the pathogenesis of inflammatory bowel disease. Am J Physiol Heart Circ Physiol. 2003;285:1791–1796.
Spalinger J, Patriquin H, Miron MC, et al. Doppler US in patients with Crohn disease: vessel density in the diseased bowel reflects disease activity. Radiology. 2000;217:787–791.
Schinzari F, Armuzzi A, De Pascalis B, et al. Tumor necrosis factor-alpha antagonism improves endothelial dysfunction in patients with Crohn’s disease. Clin Pharmacol Ther. 2008;83:70–76.
Tolstanova G, Khomenko T, Deng X, et al. Neutralizing anti-vascular endothelial growth factor (VEGF) antibody reduces severity of experimental ulcerative colitis in rats: direct evidence for the pathogenic role of VEGF. J Pharmacol Exp Ther. 2009;328:749–757.
Chidlow JH Jr, Glawe JD, Pattilllo CB, et al. VEGF164 isoform specific regulation of T-cell-dependent experimental colitis in mice. Inflamm Bowel Dis. 2011;17:1501–1512.
Jerkic M, Peter M, Ardelean D, et al. Dextran sulfate sodium leads to chronic colitis and pathological angiogenesis in Endoglin heterozygous mice. Inflamm Bowel Dis. 2010;16:1859–1870.
Scaldaferri F, Vetrano S, Sans M, et al. VEGF-A links angiogenesis and inflammation in inflammatory bowel disease pathogenesis. Gastroenterology. 2009;136:585–595.
Plevy S, Silverberg MS, Lockton S, et al. Combined Serological, Genetic, and Inflammatory Markers Differentiate Non-IBD, Crohn’s Disease, and Ulcerative Colitis Patients. Inflamm Bowel Dis. 2013;19:1139–1148.
Coriat R, Mir O, Leblanc S, et al. Feasibility of anti-VEGF agent bevacizumab in patients with Crohn’s disease. Inflamm Bowel Dis. 2011;17:1632.
Shaughnessy AF. Monoclonal antibodies: magic bullets with a hefty price tag. BMJ. 2012;345:8346.
Loriot Y, Boudou-Rouquette P, Billemont B, et al. Acute exacerbation of hemorrhagic rectocolitis during antiangiogenic therapy with sunitinib and sorafenib. Ann Oncol. 2008;19:1975.
Basu S, Nagy JA, Pal S, et al. The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat Med. 2001;7:569–574.
Sarkar C, Chakroborty D, Chowdhury UR, et al. Dopamine increases the efficacy of anticancer drugs in breast and colon cancer preclinical models. Clin Cancer Res. 2008;14:2502–2510.
Basu S, Sarkar C, Chakroborty D, et al. Ablation of peripheral dopaminergic nerves stimulates malignant tumor growth by inducing vascular permeability factor/vascular endothelial growth factor-mediated angiogenesis. Cancer Res. 2004;64:5551–5555.
Bhattacharya R, Sinha S, Yang SP, et al. The neurotransmitter dopamine modulates vascular permeability in the endothelium. J Mol Signal. 2008;3:14.
Chakroborty D, Sarkar C, Yu H, et al. Dopamine stabilizes tumor blood vessels by up-regulating angiopoietin 1 expression in pericytes and Kruppel-like factor-2 expression in tumor endothelial cells. Proc Natl Acad Sci USA. 2011;108:20730–20735.
Magro F, Vieira-Coelho MA, Fraga S, et al. Impaired synthesis or cellular storage of norepinephrine, dopamine, and 5-hydroxytryptamine in human inflammatory bowel disease. Dig Dis Sci. 2002;47:216–224.
Magro F, Fraga S, Ribeiro T, et al. Decreased availability of intestinal dopamine in transmural colitis may relate to inhibitory effects of interferon-gamma upon l-DOPA uptake. Acta Physiol Scand. 2004;180:379–386.
Magro F, Cunha E, Araujo F, et al. Dopamine D2 receptor polymorphisms in inflammatory bowel disease and the refractory response to treatment. Dig Dis Sci. 2006;51:2039–2044.
Satoh H, Sato F, Takami K, et al. New ulcerative colitis model induced by SH blockers in rats and the effects of antiinflammatory drugs on the colitis. Jpn J Pharmacol. 1997;73:299–309.
Castaneda FE, Walia B, Vijay-Kumar M, et al. Targeted deletion of metalloproteinase 9 attenuates experimental colitis in mice: central role of epithelial-derived MMP. Gastroenterology. 2005;129:1991–2008.
Patterson CE, Rhoades RA, Garcia JG. Evans blue dye as a marker of albumin clearance in cultured endothelial monolayer and isolated lung. J Appl Physiol. 1992;72:865–873.
Tolstanova G, Deng X, French SW, et al. Early endothelial damage and increased colonic vascular permeability in the development of experimental ulcerative colitis in rats and mice. Lab Invest. 2012;92:9–21.
Khomenko T, Szabo S, Deng X, et al. Suppression of early growth response factor-1 with egr-1 antisense oligodeoxynucleotide aggravates experimental duodenal ulcers. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1211–G1218.
Webster J. A comparative review of the tolerability profiles of dopamine agonists in the treatment of hyperprolactinaemia and inhibition of lactation. Drug Saf. 1996;14:228–238.
Benedetti MS, Dostert P, Barone D, et al. In vivo interaction of cabergoline with rat brain dopamine receptors labelled with [3H]N-n-propylnorapomorphine. Eur J Pharmacol. 1990;187:399–408.
Lahlou S. Involvement of spinal dopamine receptors in mediation of the hypotensive and bradycardic effects of systemic quinpirole in anaesthetised rats. Eur J Pharmacol. 1998;353:227–237.
Gomez R, Gonzalez-Izquierdo M, Zimmermann RC, et al. Low-dose dopamine agonist administration blocks vascular endothelial growth factor (VEGF)-mediated vascular hyperpermeability without altering VEGF receptor 2-dependent luteal angiogenesis in a rat ovarian hyperstimulation model. Endocrinology. 2006;147:5400–5411.
Berg DJ, Davidson N, Kuhn R, et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4+ TH1-like responses. J Clin Invest. 1996;98:1010–1020.
Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the β-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8:1223–1234.
Kilic E, Kilic U, Wang Y, et al. The phosphatidylinositol-3 kinase/Akt pathway mediates VEGF’s neuroprotective activity and induces blood brain barrier permeability after focal cerebral ischemia. FASEB J. 2006;20:1185–1187.
Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217.
Eaker EY, Bixler GB, Dunn AJ, et al. Dopamine and norepinephrine in the gastrointestinal tract of mice and the effects of neurotoxins. J Pharmacol Exp Ther. 1988;244:438–442.
Eisenhofer G, Aneman A, Friberg P, et al. Substantial production of dopamine in the human gastrointestinal tract. J Clin Endocrinol Metab. 1997;82:3864–3871.
Eldrup E, Richter EA, Christensen NJ. DOPA, norepinephrine, and dopamine in rat tissues: no effect of sympathectomy on muscle DOPA. Am J Physiol. 1989;256:284–287.
Mezey E, Eisenhofer G, Hansson S, et al. Non-neuronal dopamine in the gastrointestinal system. Clin Exp Pharmacol Physiol Suppl. 1999;26:14–22.
Kim HJ, Koh PO, Kang SS, et al. The localization of dopamine D2 receptor mRNA in the human placenta and the anti-angiogenic effect of apomorphine in the chorioallantoic membrane. Life Sci. 2001;68:1031–1040.
Sinha S, Vohra PK, Bhattacharya R, et al. Dopamine regulates phosphorylation of VEGF receptor 2 by engaging Src-homology-2-domain-containing protein tyrosine phosphatase 2. J Cell Sci. 2009;122:3385–3392.
Li ZS, Schmauss C, Cuenca A, et al. Physiological modulation of intestinal motility by enteric dopaminergic neurons and the D2 receptor: analysis of dopamine receptor expression, location, development, and function in wild-type and knock-out mice. J Neurosci. 2006;26:2798–2807.
Szabo S, Sandrock AW, Nafradi J, et al. Dopamine and dopamine receptors in the gut: their possible role in duodenal ulceration. In: Kawasaki M, et al., eds. Advances in dopamine research. Oxford: Pergamon Press; 1982:165–170.
Tian YM, Chen X, Luo DZ, et al. Alteration of dopaminergic markers in gastrointestinal tract of different rodent models of Parkinson’s disease. Neuroscience. 2008;153:634–644.
Eldrup E, Richter EA. DOPA, dopamine, and DOPAC concentrations in the rat gastrointestinal tract decrease during fasting. Am J Physiol Endocrinol Metab. 2000;279:815–822.
Asano Y, Hiramoto T, Nishino R, et al. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol. 2012;303:1288–1295.
Bryan-Lluka LJ, O’Donnell SR. Dopamine and adrenaline, but not isoprenaline, are substrates for uptake and metabolism in isolated perfused lungs of rats. Naunyn Schmiedebergs Arch Pharmacol. 1992;346:20–26.
Tarnawski A, Coron E, Mosnier JF, et al. In-vivo detection by confocal endomicroscopy of two distinct structural abnormalities in angioarchitecture and increased VP in colonic mucosa of patients with IBD in remission: mechanistic implications. Gastroenterology. 2009;136:112.
Colucci M, Cervio M, Faniglione M, et al. Intestinal dysmotility and enteric neurochemical changes in a Parkinson’s disease rat model. Auton Neurosci. 2012;169:77–86.
Tolstanova G, Khomenko T, Deng X, et al. New molecular mechanisms of the unexpectedly complex role of VEGF in ulcerative colitis. Biochem Biophys Res Commun. 2010;399:613–616.
Alvarez C, Martí-Bonmatí L, Novella-Maestre E, et al. Dopamine agonist cabergoline reduces hemoconcentration and ascites in hyperstimulated women undergoing assisted reproduction. J Clin Endocrinol Metab. 2007;92:2931–2937.
Szabo S, Horner HC, Maull H, et al. Biochemical changes in tissue catecholamines and serotonin in duodenal ulceration caused by cysteamine or propionitrile in the rat. J Pharmacol Exp Ther. 1987;240:871–878.
Horner HC, Szabo S. Differential effect of changing central and peripheral catecholamine levels in cysteamine-induced duodenal ulcer in the rat. Life Sci. 1981;29:2437–2443.
Devos D, Lebouvier T, Lardeux B, et al. Colonic inflammation in Parkinson’s disease. Neurobiol Dis. 2013;50:42–48.
Forsyth Christopher B, Kathleen M, et al. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One. 2011;6:28032.
Salat-Foix D, Tran K, Ranawaya R, et al. Increased intestinal permeability and Parkinson disease patients: chicken or egg? Can J Neurol Sci. 2012;39:185–188.
Ray A, Henke PG, Sullivan RM. Effects of intra-amygdalar dopamine agonists and antagonists on gastric stress lesions in rats. Neurosci Lett. 1988;84:302–306.
Ray A, Henke PG. The basolateral amygdala, dopamine and gastric stress ulcer formation in rats. Brain Res. 1991;558:335–338.
McKenna F, McLaughlin PJ, Lewis BJ, et al. Dopamine receptor expression on human T- and B-lymphocytes, monocytes, neutrophils, eosinophils and NK cells: a flow cytometric study. J Neuroimmunol. 2002;132:34–40.
Besser MJ, Ganor Y, Levite M. Dopamine by itself activates either D2, D3 or D1/D5 dopaminergic receptors in normal human T-cells and triggers the selective secretion of either IL-10, TNFalpha or both. J Neuroimmunol. 2005;169:161–171.
Check JH, Katsoff B, Cohen R. Novel highly effective medical treatment of severe treatment refractory Crohn’s disease using sympathomimetic amines: case report. Inflamm Bowel Dis. 2010;16:1999–2000.
Check JH, Katsoff B, Cohen R. Case report showing that a woman with ulcerative colitis refractory to standard therapy responded well to the sympathomimetic amine dextroamphetamine sulfate. Inflamm Bowel Dis. 2011;17:870–871.
Check JH, Amadi C, Kaplan H, Katsoff D. The treatment of idiopathic edema, a cause of chronic pelvic pain in women: effectively controlled chronic refractory urticaria–case reports. Clin Exp Obstet Gynecol. 2006;33:183–184.
Acknowledgments
The present study was supported by a Department of Veterans Affairs, Veterans Health Administration Merit Review Grant VAMR0710-580 and VAMR0810-877 to Zs. Sandor and S. Szabo and by U.S. Civilian Research & Development Foundation (CRDF) CREST II Junior Scientist Research Collaboration Program 09DP036-05 and the Ministry of Education and Science of Ukraine Grant 15BF036-01 to G. Tolstanova
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tolstanova, G., Deng, X., Ahluwalia, A. et al. Role of Dopamine and D2 Dopamine Receptor in the Pathogenesis of Inflammatory Bowel Disease. Dig Dis Sci 60, 2963–2975 (2015). https://doi.org/10.1007/s10620-015-3698-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-015-3698-5