Abstract
Background
The onset of acute pancreatitis (AP) is characterized by early protease activation followed by inflammation and organ damage, but the mechanisms are poorly understood.
Aims
We hypothesized that histone deacetylase (HDAC) inhibition might exert protective effects on AP and investigated the role of HDAC in trypsin activation, inflammation, and tissue damage in severe AP.
Methods
Male C57Bl/6 mice were treated i.p. with the HDAC inhibitor trichostatin A (2 mg/kg) prior to retrograde infusion of taurocholic acid (5 %) into the pancreatic duct. Serum levels of amylase and interleukin (IL)-6, pancreatic levels of macrophage inflammatory protein-2 (MIP-2) as well as tissue morphology and myeloperoxidase activity in the pancreas and lung were determined 24 h after taurocholate challenge. Trypsin activation was analyzed in isolated acinar cells. Quantitative RT-PCR was used to examine the expression of pro-inflammatory mediators in the pancreas.
Results
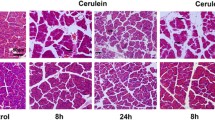
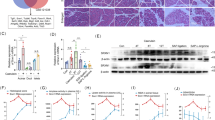
Pretreatment with trichostatin A decreased amylase levels by 70 % and protected against tissue injury in the pancreas. Moreover, HDAC inhibition reduced systemic IL-6 by more than 95 % and pulmonary myeloperoxidase activity by 75 %. Notably, inhibition of HDAC abolished taurocholate-induced gene expression of cyclooxygenase-2, MIP-2, monocyte chemotactic protein-1, IL-6, and IL-1β in the pancreas. In addition, HDAC inhibition reduced cerulein-induced trypsinogen activation in isolated acinar cells.
Conclusion
Our findings show that HDAC regulates trypsin activation, inflammation, and tissue damage in AP. Thus, targeting HDAC could serve as novel therapeutic approach in the management of severe AP.


Similar content being viewed by others
References
Regner S, Manjer J, Appelros S, Hjalmarsson C, Sadic J, Borgstrom A. Protease activation, pancreatic leakage, and inflammation in acute pancreatitis: differences between mild and severe cases and changes over the first three days. Pancreatology. 2008;8:600–607.
Awla D, Abdulla A, Zhang S, et al. Lymphocyte function antigen-1 regulates neutrophil recruitment and tissue damage in acute pancreatitis. Br J Pharmacol. 2011;163:413–423.
Hartman H, Abdulla A, Awla D, et al. P-selectin mediates neutrophil rolling and recruitment in acute pancreatitis. Br J Surg. 2012;99:246–255.
Pastor CM, Rubbia-Brandt L, Hadengue A, Jordan M, Morel P, Frossard JL. Role of macrophage inflammatory peptide-2 in cerulein-induced acute pancreatitis and pancreatitis-associated lung injury. Lab Invest. 2003;83:471–478.
Luster AD. Chemokines–chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445.
Rakonczay Z Jr, Hegyi P, Takacs T, McCarroll J, Saluja AK. The role of NF-kappaB activation in the pathogenesis of acute pancreatitis. Gut. 2008;57:259–267.
Ethridge RT, Chung DH, Slogoff M, et al. Cyclooxygenase-2 gene disruption attenuates the severity of acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology. 2002;123:1311–1322.
Bhatia M, Ramnath RD, Chevali L, Guglielmotti A. Treatment with bindarit, a blocker of MCP-1 synthesis, protects mice against acute pancreatitis. Am J Physiol. 2005;288:G1259–G1265.
Norman JG, Fink GW, Denham W, et al. Tissue-specific cytokine production during experimental acute pancreatitis. A probable mechanism for distant organ dysfunction. Dig Dis Sci. 1997;42:1783–1788.
Spencer VA, Davie JR. Role of covalent modifications of histones in regulating gene expression. Gene. 1999;240:1–12.
Wu C. Chromatin remodeling and the control of gene expression. J Biol Chem. 1997;272:28171–28174.
Lane AA, Chabner BA. Histone deacetylase inhibitors in cancer therapy. J Clin Oncol. 2009;27:5459–5468.
Marumo T, Hishikawa K, Yoshikawa M, Hirahashi J, Kawachi S, Fujita T. Histone deacetylase modulates the proinflammatory and -fibrotic changes in tubulointerstitial injury. Am J Physiol. 2010;298:F133–F141.
Endres M, Meisel A, Biniszkiewicz D, et al. DNA methyltransferase contributes to delayed ischemic brain injury. J Neurosci. 2000;20:3175–3181.
Zhao TC, Du J, Zhuang S, Liu P, Zhang LX. HDAC inhibition elicits myocardial protective effect through modulation of MKK3/Akt-1. PLoS ONE. 2013;8:e65474.
Zhang L, Jin S, Wang C, Jiang R, Wan J. Histone deacetylase inhibitors attenuate acute lung injury during cecal ligation and puncture-induced polymicrobial sepsis. World J Surg. 2010;34:1676–1683.
Laukkarinen JM, Van Acker GJ, Weiss ER, Steer ML, Perides G. A mouse model of acute biliary pancreatitis induced by retrograde pancreatic duct infusion of Na-taurocholate. Gut. 2007;56:1590–1598.
VanGuilder HD, Vrana KE, Freeman WM. Twenty-five years of quantitative PCR for gene expression analysis. Biotechniques. 2008;44:619–626.
Suzuki K, Ota H, Sasagawa S, Sakatani T, Fujikura T. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal Biochem. 1983;132:345–352.
Schmidt J, Rattner DW, Lewandrowski K, et al. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215:44–56.
Saluja AK, Bhagat L, Lee HS, Bhatia M, Frossard JL, Steer ML. Secretagogue-induced digestive enzyme activation and cell injury in rat pancreatic acini. Am J Physiol. 1999;276:G835–G842.
Kawabata S, Miura T, Morita T, et al. Highly sensitive peptide-4-methylcoumaryl-7-amide substrates for blood-clotting proteases and trypsin. Eur J Biochem. 1988;172:17–25.
Rubartelli A, Cozzolino F, Talio M, Sitia R. A novel secretory pathway for interleukin-1 beta, a protein lacking a signal sequence. EMBO J. 1990;9:1503–1510.
Mithofer K, Fernandez-del Castillo C, Rattner D, Warshaw AL. Subcellular kinetics of early trypsinogen activation in acute rodent pancreatitis. Am J Physiol. 1998;274:G71–G79.
Abdulla A, Awla D, Thorlacius H, Regner S. Role of neutrophils in the activation of trypsinogen in severe acute pancreatitis. J Leukoc Biol. 2011;90:975–982.
Acknowledgments
This work was supported by grants from the Swedish Medical Research Council (2012-3685), Crafoord Foundation, Einar and Inga Nilsson Foundation, Harald and Greta Jaensson Foundation, Greta and Johan Kock Foundation, Magnus Bergvall Foundation, Mossfelt Foundation, Nanna Svartz Foundation, Ihre foundation, Schyberg foundation, Mag-tarm fonden, Ruth and Richard Julin Foundation, Malmö Hospital Cancer Foundation, Malmö Hospital Foundation, Malmö University Hospital and Lund University.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hartman, H., Wetterholm, E., Thorlacius, H. et al. Histone Deacetylase Regulates Trypsin Activation, Inflammation, and Tissue Damage in Acute Pancreatitis in Mice. Dig Dis Sci 60, 1284–1289 (2015). https://doi.org/10.1007/s10620-014-3474-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-014-3474-y