Abstract
Background
Fulminant hepatic failure (FHF) is a devastating syndrome, which sometimes results in death or liver transplantation, in which inflammation would aggravate the development of fetuin-A which would act as an anti-inflammatory factor and may be an available approach to attenuate FHF.
Aims
The purpose of this study was to investigate the effects of fetuin-A on d-galactosamine/lipopolysaccharide (d-GalN/LPS)-induced liver failure in mice.
Methods
A mouse model of FHF induced by d-GalN/LPS was established and fetuin-A was injected intraperitoneally prior to d-GalN/LPS treatment. At different time points after d-GalN/LPS intervention, serum TNF-α and IL-6 levels were measured by ELISA. Fetuin-A mRNA and protein expression in liver tissues was assessed by RT-PCR, Western blotting and immunohistochemical staining. Besides, an observation of liver tissue injury, the apoptosis of hepatocytes, was analyzed by TUNEL assay.
Results
Expression of fetuin-A mRNA and protein in liver tissue were significantly and gradually decreased after d-GalN/LPS administration. A pre-intervention of exogenous fetuin-A significantly improved the liver function, decreased TNF-α and IL-6 expression in peripheral blood, and liver tissue inhibited hepatocyte apoptosis responded to d-GalN/LPS induction so as to decrease the mortality rates of FHF mouse. Meanwhile, fetuin-A was negatively correlated with the hepatic pathological score and TNF-α protein staining in FHF mouse.
Conclusions
An intraperitoneal injection of fetuin-A attenuates d-GalN/LPS-induced FHF in mice. Fetuin-A might be a protective agent of liver damage partly through inhibiting liver inflammatory response and hepatocyte apoptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fulminant hepatic failure (FHF) is a condition with rapid deterioration of hepatocyte function resulting in hepatic encephalopathy and/or coagulopathy in the patients [1]. This is a devastating syndrome, which results in death or the need for liver transplantation in over 50 % of the cases [2]. Urgent liver transplantation has become the standard care for most FHF patients in some countries where FHF survival rates have shown progressive and substantial improvement with 1-year survival exceeding 80 % [2, 3]. However, in some other countries, access to liver transplantation and other extracorporeal liver-assist devices is severely limited. On the other hand, FHF also has a positive reversible potential, where survivors recover completely without any sequelae. Accordingly, if the individual can be supported properly throughout the acute event, recovery will follow the rapid regeneration of hepatocytes. Thus, it is of important clinical significance to find a more effective approach to treat patients with FHF when a liver transplantation is not available.
The mechanisms of FHF are quite complex and still not fully defined. It is generally acknowledged that immunologic injury, hypoxic ischemic lesion and endotoxemia are the three main causes. Normally, the transition from stable cirrhosis to FHF is caused by systemic inflammatory response, characterized by a predominantly proinflammatory cytokine profile [4]. Therefore, removal of proinflammatory cytokines such as TNF-α in plasma might be beneficial to FHF patients.
Fetuin-A (formerly named as α2-Heremans-Schmid glycoprotein, AHSG) is an abundant serum protein that is exclusively produced by the liver, tongue and placenta [5, 6]. As a glycoprotein, fetuin-A carries two N-linked and three O-linked oligosaccharide chains that terminate with sialic acid residues and can bind biogenic cationic ions (e.g., Ca2+) and other anti-inflammatory molecules (e.g., spermine) [7, 8]. Accordingly, fetuin-A has been proposed as an endogenous inhibitor of pathological mineralization or calcification [9–12] and an opsonin of cationic molecules (such as spermine) [7]. During FHF, it was found that liver would orchestrate a defensive response to inflammation by altering the synthesis and release of “acute phase proteins” (APPs), such as fetuin-A [13]. Data showed that circulating fetuin-A level decreased significantly (about 30–50 %) during the acute phase of inflammation and injury [7], while some classic proinflammatory cytokines such as TNF-α, IL-1, and IL-6 increased [14]. Moreover, higher concentrations of fetuin-A would act as an anti-inflammatory factor, inhibiting bacterial endotoxin-induced production of proinflammatory mediators (such as TNF, IL-1, and nitric oxide) in macrophage cultures effectively implying that fetuin-A is associated with inflammation activity [15]. In an animal model of arrageenan-induced paw edema, intraperitoneal administration of fetuin-A attenuated local TNF production and inflammation [16]. In early cerebral ischemic injury, fetuin-A played a protective role partly by attenuating the brain inflammatory response [17]. Therefore, fetuin-A may be an available approach to control serious inflammation response in FHF. To verify our hypothesis, we established a mouse model of FHF to explore roles of fetuin-A in the pathogenesis of FHF.
d-GalN is a commonly used hepatotoxic drug in the FHF model that induces dose-dependent liver injury while LPS play an important role in liver injury, and the mechanism is as follows: (1) Through inflammatory factors, LPS can damage the completeness of the vascular endothelial cell and results in microvascular damage, formation of microthrombus and leads to intrahepatic hemorrhage and hepatocyte necrosis. (2) LPS promotes secretions of multiple pro-inflammatory cytokines by Hepatic stellat cells, e.g. TNF-α, IL-1β and IL-6 etc. Combination of d-GalN and LPS created a better simulation of FHF and has been widely used recently. In our previous work, we established a stable and effective mouse model of fulminant hepatic failure (FHF) induced by d-GalN (600 mg/kg), and LPS (0.5 mg/kg) through factorial experiment [18–20].
Methods
Animal Studies
Eight-week-old BALB/c mice (SPF, female, 28–30 g) were obtained from the Hunan Agro-Techniques University Laboratories (Changsha, China). To induce acute liver failure, the mice (except for the controls) were injected intraperitoneally (i.p.) with d-GalN (600 mg/kg, Department of Biomedical Engineering, Chongqing Medical College, China) and LPS (0.5 mg/kg; Escherichia coli, Sigma, St. Louis, MO) dissolved in phosphate-buffered saline (PBS). Fetuin-A (100 mg/kg body weight, ICN, Sigma) was injected intraperitoneally 1 h prior to d-GalN/LPS treatment.
The mice were then randomly assigned into five groups (n = 6 for each): Blank control: no intervention; FHF group: 600 mg/kg d-GalN with 0.5 mg/kg LPS, i.p.; Sham group: physiologic saline with 600 mg/kg d-GalN and 0.5 mg/kg LPS, i.p.; Fetuin-A protection group: 100 mg/kg fetuin-A with 600 mg/kg d-GalN and 0.5 mg/kg LPS, i.p.; Anti-fetuin-A group: 100 mg/kg rabbit-anti-mouse fetuin-A with 600 mg/kg d-GalN and 0.5 mg/kg LPS, i.p.
For continuous stimulation in vivo, BALB/c mice were maintained under 12-h light/dark cycles with a regular diet of LPS (0.5 mg/g body weight), d-GalN or bovine serum albumin (100 mg/g body weight), intraperitoneal bolus of bovine fetuin-A (100 mg/kg body weight), rabbit anti-mouse fetuin-A and comparable amount of sterile saline respectively (except for blank control groups).
At selected time points of the 3rd, 6th and 9th hour after d-GalN/LPS treatment, the mice were anesthetized and blood samples were collected and the animals were sacrificed. The liver tissues were harvested by resections and were either stored in liquid nitrogen for RT-PCR and Western blot or fixed in 10 % formaldehyde for immunohistochemistry and TUNEL analysis. The mortality rate of the mice was observed 24 h after the experiment began in another parallel experiment group. Furthermore, to observe the potential toxicity and side effects of fetuin-A in cardiac, renal and hepatic tissue, fetuin-A (100 mg/Kg) was injected intraperitoneally in normal mouse (n = 6), the animals were sacrificed and the tissues of liver, heart and kidney were harvested by resections after 24 h. The pathological changes were observed in a general way. The Animal Use Committee of Xiangya Hospital approved all protocols for treating animals and all mice were treated humanely during the whole study period.
RT-PCR
For quantification of fetuin-A mRNA expression in mouse liver tissue, total RNA was isolated and the first strand cDNA was synthesized by the Advantage RT-for-PCR kit (BD Biosciences, Palo Alto, CA). The primers for mouse fetuin-A (GenBank Accession No.: BC012678) as: 5′-TCGGAGTGGTGTATGAGATGGAAG-3′ (sense) and 5′-GGCAGTGTTGACGGTGTGGAC-3′ (antisense) were synthesized by Boya Company (Shanghai, China). The regular PCR producing a 281 bp fragment was carried out and glyceraldehydes 3-phosphate dehydrogenate (GAPDH, GenBank Accession No.: BC029618) was used as an internal control.
Western Blotting
Mouse fetuin-A-specific antibodies were obtained from Dr. Haichao Wang (USA). Goat anti-rabbit aquaporin-2 antibodies were obtained from Santa Cruz (Santa Cruz, CA). After SDS-PAGE, the proteins were transferred onto PVDF membrane and hybridized with specific primary antibodies, and subsequently were incubated with HRP-conjugated sheep anti-mouse IgG. Bands were visualized using the ECL kit (Amersham, Piscataway, NJ) per the manufacturer’s instruction [21, 22].
Histological Assessment and Immunohistochemical (IHC) Staining
Four μm-thick formalin-fixed liver tissue sections were incubated with the primary antibody, subsequently incubated with the secondary antibody conjugated with streptavidin–biotin–peroxidase complex (Zhongshan Goldenbridge Biotechnology, Beijing, China) and a color reaction was developed using 3,3-diaminobenzidine tetrahydrochloride (Sigma). The negative control slides were probed with normal rabbit serum under the same experimental conditions.
The histological characteristics of liver tissues were scored by Ishak Classification [23]. In each case, the immunoreactivity was determined in five random fields and the IHC scoring was made independently by two experienced pathologists. The images were analyzed using Image J, a digital image processing system.
TUNEL Assays
Apoptosis of hepatocytes was detected using terminal deoxynucleotidyl transferasemediated dUTP nick-end labeling (TUNEL, red fluorescence) kit (Doctor Biotechnology, Wuhan, China). Images were observed on a Nikon Eclipse E800 fluorescent microscope. The average number of apoptotic cells per high-power field (200× magnification) was calculated in ten random visual fields for each sample and six samples for each time point, and the apoptotic index (AI) of each specimen was scored.
Liver Function Tests and Cytokine Quantification
The serum concentrations of TNF-α and IL-6 in mice were measured by commercial enzyme-linked immunosorbent assays (ELISA, Bio Vendor Laboratory Medicine, Brno, Czech Republic). The level of alanine aminotransferase (ALT) in mice serum was determined using an automatic biochemical analyzer (HITACHI-7170A, Hitachinaka City, Japan).
Statistical Analysis
The continuous data were presented as mean ± SD. Using SPSS 15.0 software (SPSS, Chicago, IL), one-way ANOVA tests were employed for comparison among all groups, and Student–Newman–Keuls test was subsequently applied to compare the statistical difference between two groups. Meanwhile, multivariate linear regression analysis was performed using log-transformed data and Pearson’s correlation coefficient to test the correlation between the relevant covariates (such as pathological score, grey scales of TNF-α and fetuin-A, and apoptotic index). All tests were two-sided and p value <0.05 was considered to be statistically significant.
Results
Liver Injury, Inflammation, and Hepatocyte Apoptosis in FHF Mouse
At the third hour after d-GalN/LPS administration, the mice in the FHF group exhibited slightly disordered liver structure, active blood sinusoid response, ballooning degeneration of hepatocytes, spot necrosis around the central veins of the hepatic lobules, scattered inflammatory cell infiltration, and eosinophilic bodies. After 6 h, the liver tissues showed aggravated structural disorder, active blood sinusoid response, lamellar necrosis, eosinophilic hepatocytes, moderate to severe inflammatory cell infiltration and numerous apoptotic bodies. After 9 h, the tissue showed widespread necrosis, abundant erythrocyte obturation, piles of inflammatory cell infiltration, numerous apoptotic bodies and eosinophilic bodies (Fig. 1a). The Ishak score showed that the injury in liver tissue aggravated over time (Table 1). The IHC staining of TNF-α staining in liver tissue reached a maximum at the ninth hour after d-GalN/LPS injection in the FHF group (Fig. 1b). The peak of apoptotic index of hepatocytes was observed in the sixth hour in the FHF mouse model (Figs. 1c, 3e).
Hepatic injury, TNF-α expression, and apoptosis assessment in the liver tissues of FHF mouse. a Pathological characteristics of the hepatic tissues in the FHF mouse model at different time points after d-GalN/LPS induction (×400). The hepatic injury, such as necrosis, structural modification, and inflammatory response, was aggravated in a time-dependent manner. b Immunohistochemistry (IHC) staining of TNF-α in hepatic tissues at the 0, third, sixth, and ninth hours after d-GalN/LPS induction in the FHF mouse (×400). Highly concentrated staining of TNF-α in brown color was obvious both in the hepatocyte cytoplasm and on the cell membrane during the ninth hour. c Apoptosis of hepatocytes in FHF mouse (×400): the stained apoptotic cells were indicated with arrowheads
Fetuin-A Expression in Liver Tissue was Reduced by d-GalN/LPS Induction in FHF Mouse
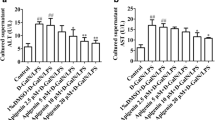
In FHF mouse model, both mRNA and protein expression (Fig. 2a, b) of fetuin-A in liver tissue were decreased significantly and gradually from the sixth to the ninth hour after d-GalN/LPS administration. Meanwhile, the significantly decreased fetuin-A protein expression, which was induced by d-GalN/LPS, was further identified by IHC staining in the FHF group (Fig. 2c).
Expression of fetuin-A mRNA and protein in d-GalN/LPS-induced FHF mouse. a, b mRNA and protein expression of fetuin-A in the liver tissues was distinctly decreased in a time-dependent manner after d-GalN/LPS induction. c, d IHC scores of fetuin-A staining, which was located in hepatocyte cytoplasm and on cell membrane of FHF mouse, were significantly decreased in a time-dependent manner after d-GalN/LPS induction
Exogenous Fetuin-A Decreased the Mortality of FHF Mouse Caused by d-GalN/LPS Injection
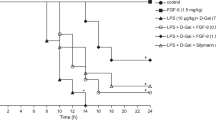
At the 24th hour of d-GalN/LPS treatment, 83 % (5/6) of the mice in the FHF group and 100 % (6/6) of the mice in the groups of sham and anti-fetuin-A were dead. In contrast, only 16.7 % (1/6) of the mice in the fetuin-A protection group died. The administration of fetuin-A has significantly decreased the mortality rates of the mouse with FHF induced by d-GalN/LPS (Fig. 3a).
Effects of fetuin-A on proinflammatory factors, apoptosis, and liver function in FHF mouse. a Fatality rate in different experimental groups after 24 h of induction. b Serum concentration of ALT in different experimental groups. c Serum concentration of proinflammatory factors of TNF-α and IL-6 in different experimental groups. d Relative IHC scores of fetuin-A in the liver tissues of different groups. e Relative IHC scores of TNF-α in the liver tissues of different groups. f Apoptosis index (AI) of hepatocytes after d-GalN/LPS intervention in different groups
Fetuin-A Improved Liver Function in FHF Mouse
Following d-GalN/LPS injection, the serum ALT significantly increased in a time-dependent manner in FHF mice (data not shown). At the ninth hour after d-GalN/LPS induction, serum ALT levels in groups of FHF (291,370.76 ± 29,241.68 nkat/L), sham (265,975.30 ± 26,652.21 nkat/L) and anti-fetuin-A (301,256.25 ± 6,258.38 nkat/L) were significantly higher than that in the groups of blank (1,055.21 ± 84.68 nkat/L) (p < 0.0001, p < 0.0001, p < 0.0001, respectively) and fetuin-A protection (31,585.15 ± 4,086.35 nkat/L) (p < 0.0001, p < 0.0001, p < 0.0001, respectively) (Fig. 3b).
Fetuin-A Attenuated Hepatic Inflammatory Response to d-GalN/LPS Intervention in FHF Mouse
The peak of serum proinflammatory factors was observed 3 h after d-GalN/LPS injection. As shown in Fig. 3c, serum TNF-α in fetuin-A protection group (233.87 ± 48.03 pg/mL) was significantly lower than that in both groups of FHF (515.48 ± 46.31 pg/mL, p = 0.034), sham (545.39 ± 62.14 pg/mL, p = 0.001), and anti-fetuin-A (692.39 ± 310.59 pg/mL, p < 0.0001) groups. Compared with blank control (9.36 ± 6.28 pg/mL), serum TNF-α level in the anti-fetuin-A group with an antagonism against endogenous fetuin-A in mouse liver was significantly higher (p < 0.0001, respectively).
Meanwhile, the serum level of IL-6 in the fetuin-A protection group (546.73 ± 56.42 pg/mL) was significantly lower than that in both the FHF (1,093.93 ± 153.15 pg/mL, p < 0.0001), sham (1,189.26.93 ± 134.72 pg/mL, p < 0.0001) and anti-fetuin-A groups (1,034.61 ± 266.12 pg/mL, p < 0.0001), indicating that an intervention of fetuin-A significantly decreased the serum level of IL-6 in the mouse with FHF. Similar to serum TNF-α comparison, an antagonism against endogenous fetuin-A of mouse liver in the anti-fetuin-A group significantly increased serum IL-6 levels compared with blank control (56.87 ± 34.98 pg/mL, p < 0.0001, respectively) (Fig. 3c). Thus, an administration of fetuin-A significantly decreased the serum concentrations of proinflammation factors, such as IL-6 and TNF-α in the mouse with FHF.
Fetuin-A and TNF-α Protein Expression in the Liver Tissues of FHF Mouse
Since d-GalN/LPS intervention inhibited fetuin-A expression in a time-dependent manner and reached the lowest level at the ninth hour in the liver tissue of FHF mouse (Fig. 2), the ninth hour was subsequently chosen as the check point to compare fetuin-A and TNF-α expression levels among different experimental groups (n = 6). As shown in Fig. 3d, the relative IHC scores of fetuin-A in the groups of the blank control (114.68 ± 0.67,) and fetuin-A protection (113.29 ± 1.98) were significantly higher than that in the groups of FHF (110.89 ± 0.86, p < 0.0001, p < 0.0001, respectively), sham (110.45 ± 0.67, p < 0.0001, p < 0.0001, respectively) and anti-fetuin-A (109.77 ± 1.01, p < 0.0001, p < 0.0001, respectively) groups.
After d-GalN/LPS injection in mouse, IHC staining of TNF-α in the liver tissue of FHF mouse was gradually increased and reached the highest level at the sixth hour. The relative IHC staining intensities of TNF-α in the groups of the blank control (112.14 ± 3.07) and fetuin-A protection (111.95 ± 2.59) were significantly lower than that in the groups of both FHF (117.01 ± 3.42, p = 0.002, p < 0.0001 respectively), sham (116.45 ± 2.60, p = 0.002, p < 0.0001 respectively) and anti-fetuin-A (115.55 ± 0.85, p = 0.016, p = 0.0004, respectively) (Fig. 3e). An intraperitoneal injection prior to d-GalN/LPS treatment effectively attenuates TNF-α expression in the liver tissues of the FHF mouse. Moreover, TNF-α expression in the anti-fetuin-A group was significantly higher than that in the fetuin-A protection group, indicating that an antagonism against endogenous fetuin-A promoted liver inflammation in FHF mouse.
Fetuin-A Inhibited the Apoptosis of Hepatocytes in FHF Mouse
After d-GalN/LPS injection, an obvious hepatocyte apoptosis was observed in the liver tissue of FHF mouse (Fig. 2f). Apoptosis was found occasionally in the blank control (0.10 ± 0.32) group. However, the hepatocyte apoptosis index (AI) in the groups of FHF (23.10 ± 7.39, p < 0.0001), anti-fetuin-A (13.50 ± 3.47, p < 0.0001) and fetuin-A protection (2.20 ± 2.97) was statistically significant (Fig. 3f). Thus, the interference of fetuin-A significantly inhibited hepatocyte apoptosis induced by d-GalN/LPS treatment in FHF mouse.
Toxicity and Side Effects of Fetuin-A on Normal Mouse Liver, Kidney and Heart
As a potential therapeutic drug for FHF, fetuin-A at the experimental dosage was further evaluated in terms of potential toxicity and side effects in cardiac, renal and hepatic tissues. As shown in Fig. 4, all the histological appearances of cardiac, renal and hepatic tissues were normal after fetuin-A injection at 100 mg/kg for 24 h (Fig. 4).
Correlation Among Hepatic Pathological Scores, Expression of Fetuin-A, TNF-α, and Hepatocyte Apoptosis
For a multiple linear regression analysis, the hepatic pathological scores of FHF mouse were adopted as the dependent variable (Y), while the relative IHC staining scores of fetuin-A and TNF-α and hepatic AI were respectively used as the independent variables X1, X2, X3; a regression equation was constructed as: Y = −4.388 − 2.682 X1 + 0.073 X2 + 0.032 X3 (R² = 0.603; F test: F = 7.075, p = 0.004).
Moreover, a correlation test was carried out among these four aforementioned indices. The Pearson’s contiguous coefficients showed that the relative IHC score of fetuin-A was negatively correlated with the pathological score, while the relative IHC score of TNF-α was positively correlated with the pathological score and the relative IHC scores of fetuin-A and TNF-α were negatively correlated with each other (Table 2).
Discussion
In response to infection or injury, the liver re-prioritizes the synthesis and systemic release of many APPs, one of which is fetuin-A [24, 25]. In the present study, we found that fetuin-A expression in liver tissue was time-dependently decreased during FHF. Meanwhile, fetuin-A expression was negatively correlated with TNF-α in the liver of FHF mouse. During FHF, the severe necrosis and apoptosis directly reduced the number of hepatocytes and also affected the functions of living cells so that fetuin-A produced by hepatocytes was decreased and the hepatic functions were severely injured. An exogenous injection of fetuin-A prior to d-GalN/LPS intervention could lower the circulating levels of TNF-α and IL-6, decrease hepatic apoptosis, improve liver function and eventually lower the fatality rate of FHF mice. With regard to the safety of intraperitoneal injection of fetuin-A, our data did not find obvious pathological damage to mouse liver, kidney and heart tissues at the dose of 100 mg/kg. As a result, an intraperitoneal administration of fetuin-A attenuated d-GalN/LPS-induced liver failure in mice.
Although the mortality and serum ALT levels of FHF mouse were elevated by a certain dose of anti-fetuin-A (100 mg/kg) to interfere the endogenous fetuin-A, no statistical significance was found between the groups of FHF and anti-fetuin-A (100 mg/kg). The possible reasons may be the abundant endogenous fetuin-A in mice (about 500 mg/kg), so that 100 mg/kg of anti-fetuin-A was not enough for antagonism. Secondly, an extended sample size might be better for exhibiting the statistical differences among different experimental groups.
The hepatic apoptosis was obvious in our FHF mouse model. When apoptosis occurred in a large amount of hepatocytes, apoptotic cells were not endocytized promptly by phagocytes. The cellular contents were delivered after these cells were distended and split, which stimulated neutrophilic granulocytes to release and recruit inflammatory factors. Then inflammatory response was generated, aggravated and prolonged. Previous studies have shown that fetuin-A played an important role in recognition and clearance of apoptotic cells [26], inhibited the apoptosis of vascular smooth muscle cells and reduced the cleavage of caspases 3, 8, and 9 into their active subunits [27]. So fetuin-A could be a protective agent for tissue injury through apoptosis inhibition. Moreover, we found that fetuin-A could also decrease hepatic apoptosis indirectly by depressing TNF-α, which could induce hepatic apoptosis.
On the other hand, fetuin-A was documented to be critical in phagocytosis [28] and inhibit the inflammatory response of macrophages after phagocytosis [29]. In our study, multiple linear regression analysis demonstrated that fetuin-A, which was correlated with TNF-α closely but oppositely, was probably a positive regulator of pathological damage of hepatic tissue in FHF mouse. The decreased fetuin-A in liver tissue ensures a rigorous innate immune response manifested by excessive accumulation of proinflammatory mediators. Supplementation with exogenous fetuin-A could tilt the balance towards inhibiting the release of activated TNF-α and IL-6.
Fetuin-A is an abundant serum protein that is exclusively produced by the liver, tongue and placenta. It has biological homology, high affinity and no significant side effects. Furthermore, administration of fetuin-A confers protection against fulminant hepatic failure in the FHF mouse model, and thus calls for need to further explore its therapeutic potential for the clinical management of acute liver failure.
In summary, the present study demonstrates fetuin-A as a negative APP in FHF. Fetuin-A exerts an important protective role against FHF by counter-regulating systemic accumulation of proinflammatory mediators.
References
O’Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet. 1993;342:273–275.
Ostapowicz G, Fontana RJ, Schiødt FV, et al. U.S. Acute Liver Failure Study Group. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954.
Wigg AJ, Gunson BK, Mutimer DJ. Outcomes following liver transplantation for seronegative acute liver failure: experience during a 12-year period with more than 100 patients. Liver Transpl. 2005;11:27–34.
Rolando N, Wade J, Davalos M, et al. The systemic inflammatory response syndrome in acute liver failure. Hepatology. 2000;32:734–739.
Dziegielewska KM, Brown WM, Casey SJ, et al. The complete cDNA and amino acid sequence of bovine fetuin. Its homology with alpha 2HS glycoprotein and relation to other members of the cystatin superfamily. J Biol Chem. 1990;265:4354–4357.
Denecke B, Graber S, Schafer C, et al. Tissue distribution and activity testing suggest a similar but not identical function of fetuin-B and fetuin-A. Biochem J. 2003;376:135–145.
Wang H, Zhang M, Soda K, et al. Fetuin protects the fetus from TNF. Lancet. 1997;350:861–862.
Suzuki M, Shimokawa H, Takagi Y, et al. Calcium-binding properties of fetuin in fetal bovine serum. J Exp Zool. 1994;270:501–507.
Schinke T, Amendt C, Trindl A, et al. The serum protein alpha2-HS glycoprotein/fetuin inhibits apatite formation in vitro and in mineralizing calvaria cells. A possible role in mineralization and calcium homeostasis. J Biol Chem. 1996;271:20789–20796.
Szweras M, Liu D, Partridge EA, et al. Alpha 2-HS glycoprotein/fetuin, a transforming growth factor-beta/bone morphogenetic protein antagonist, regulates postnatal bone growth and remodeling. J Biol Chem. 2002;277:19991–19997.
Schafer C, Heiss A, Schwarz A, et al. The serum protein alpha 2-Heremans–Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357–366.
Ketteler M, Bongartz P, Westenfeld R, et al. Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: a cross-sectional study. Lancet. 2003;361:827–833.
Christie DL, Dziegielewska KM, Hill RM. Saunders NR Fetuin: the bovine homologue of human alpha 2HS glycoprotein. FEBS Lett. 1987;214:45–49.
Daveau M, Christian D, Julen N, et al. The synthesis of human alpha-2-HS glycoprotein is down-regulated by cytokines in hepatoma HepG2 cells. FEBS Lett. 1988;241:191–194.
Wang H, Zhang M, Bianchi M, et al. Fetuin (alpha2-HS-glycoprotein) opsonizes cationic macrophagedeactivating molecules. Proc Natl Acad Sci. 1998;95:14429–14434.
Ombrellino M, Wang H, Yang H, et al. Fetuin, a negative acute phase protein, attenuates TNF synthesis and the innate inflammatory response to carrageenan. Shock. 2001;15:181–185.
Wang H, Li W, Zhu S, et al. Peripheral administration of fetuin-A attenuates early cerebral ischemic injury in rats. J Cereb Blood Flow Metab. 2010;30:493–504.
Lian LH, Jin X, Wu YL, et al. Hepato protective effects of Sedum sarmentosum on D-galactosamine/lipopolysaccharide-induced murine fulminant hepatic failure. J Pharmacol Sci. 2010;114:147–157.
Gong X, Luo F, Zhang L, et al. Tetrandrine attenuates lipopolysaccharide-induced fulminant hepatic failure in D-galactosamine-sensitized mice. Int Immunopharmacol. 2010;10:357–363.
Avlas O, Pappo O, Zilbermints V, et al. Reduced hepatic injury in toll-like receptor 4-deficient mice following D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure. Cell Physiol Biochem. 2012;29:41–50.
Li W, Zhu S, Li J, et al. A hepatic protein, fetuin-A, occupies a protective role in lethal systemic inflammation. PLoS One. 2011;6:e16945.
Wang H, Li W, Zhu S, et al. Peripheral administration of fetuin-A attenuates early cerebral ischemic injury in rats. J Cereb Blood Flow Metab. 2009;30:493–504.
Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699.
Faulkner L, Altmann DM, Ellmerich S, et al. Sexual dimorphism in superantigen shock involves elevated TNF-alpha and TNF-alpha induced hepatic apoptosis. Am J Respir Crit Care Med. 2007;176:473–482.
Li Wei, Zhu Shu, Li Jianhua, et al. A hepatic protein, fetuin-A, occupies a protective role in lethal systemic inflammation. J PLoS ONE. 2011;6:16945.
Weikert C, Stefan N, Schulze MB, et al. Plasma fetuin-A levels and the risk of myocardial infarction and ischemic stroke. Circulation. 2008;118:2555–2562.
Li LJ, Huang JR, Yang Q. A report from the 2001 international symposium on artificial liver. Zhonghua anzangbing Zazhi. 2001;9:383–384.
Nauta AJ, Daha MR, van Kooten C, et al. Recognition and clearance of apoptotic cells: a role for complement and pentraxins. Trends Immunol. 2003;24:148–154.
Reynolds JL, Skepper JN, McNair R, et al. Multifunctional roles for serum protein fetuin-A in inhibition of human vascular smooth muscle cell calcification. J Am Soc Nephrol. 2005;16:2920–2930.
Acknowledgments
This study was funded by the “Specialized Research Fund for the Doctoral Program of Higher Education of China,” Grant Number (200805331166) and the “Natural Science Foundation of Hunan Province of China,” Grant number (14JJ3043).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Zhang, P., Shen, H., Huang, J. et al. Intraperitoneal Administration of Fetuin-A Attenuates d-Galactosamine/Lipopolysaccharide-Induced Liver Failure in Mouse. Dig Dis Sci 59, 1789–1797 (2014). https://doi.org/10.1007/s10620-014-3071-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-014-3071-0