Abstract
Background
Chronic hepatitis C (HCV) is a significant risk factor for cirrhosis and subsequently hepatocellular carcinoma (HCC). HCV patients with cirrhosis are screened for HCC every 6 months. Surveillance for progression to cirrhosis and consequently access to HCC screening is not standardized. Liver biopsy, the usual test to determine cirrhosis, carries a significant risk of morbidity and associated mortality. Transient ultrasound elastography (fibroscan) is a non-invasive test for cirrhosis.
Purpose
This study assesses the cost effectiveness of annual surveillance for cirrhosis in patients with chronic HCV and the effect of replacing biopsy with fibroscan to diagnose cirrhosis.
Method
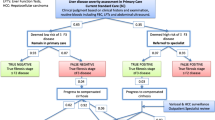
A Markov decision analytic model simulated a hypothetical cohort of 10,000 patients with chronic HCV initially without fibrosis over their lifetime. The cirrhosis surveillance strategies assessed were: no surveillance; current practice; fibroscan in current practice with biopsy to confirm cirrhosis; fibroscan completely replacing biopsy in current practice (definitive); annual biopsy; annual fibroscan with biopsy to confirm cirrhosis; annual definitive fibroscan.
Results
Our results demonstrate that annual definitive fibroscan is the optimal strategy to diagnose cirrhosis. In our study, it diagnosed 20 % more cirrhosis cases than the current strategy, with 549 extra patients per 10,000 accessing screening over a lifetime and, consequently, 76 additional HCC cases diagnosed. The lifetime cost is £98.78 extra per patient compared to the current strategy for 1.72 additional unadjusted life years. Annual fibroscan surveillance of 132 patients results in the diagnosis one additional HCC case over a lifetime. The incremental cost-effectiveness ratio for an annual definitive fibroscan is £6,557.06/quality-adjusted life years gained.
Conclusion
Annual definitive fibroscan may be a cost-effective surveillance strategy to identify cirrhosis in patients with chronic HCV, thereby allowing access of these patients to HCC screening.





Similar content being viewed by others
References
London: Health Protection Agency. Hepatitis C in the UK 2012. London: Health Protection Agency, Colindale.
Argeudas MR, Chen VK, Eloubeidi MA, Fallon MB. Screening for hepatocellular carcinoma in patients with hepatitis C cirrhosis: a cost-utility analysis. Am J Gastroenterol. 2003;98:679–690.
Lemon SM, McGiven DR. Is hepatitis C virus carcinogenic? Gastroenterology. 2012;142:1274–1278.
Bedossa P, Poynard T and the French METAVIR Cooperative Study Group. An algorithm for grading activity in chronic hepatitis C. Hepatology. 1996;24:289–293.
Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ. Cost-effectiveness of treatment for chronic hepatitis C infection in an evolving patient population. J Am Med Assoc. 2003;290:228–237.
Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48:418–431.
Bennett WG, Inoue Y, Beck JR, Wong JB, Pauker SG, Davis GL. Estimates of the cost effectiveness of a single course of interferon-alpha 2b in patients with histologically mild chronic hepatitis C. Ann Intern Med. 1997;127:855–865.
Freeman AJ, Dore GJ, Law MG, et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology. 2001;34:809–816.
Grishchenko M, Grieve RD, Sweeting MJ, et al and the Trent HCV Study group. Cost-effectiveness of pegylated interferon and ribivarin for patients with chronic hepatitis C treated in routine clinical practice. Int J Technol Assess Health Care. 2009;25:171–180.
Townsend R, McEwan P, Kim R, Yuan Y. Structural frameworks and key model parameters in cost-effectiveness analyses for current and future treatments of chronic hepatitis C. Value Health. 2011;14:1068–1077.
Fattovich G, Giustina G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463–472.
Benvegnu L, Alberti A. Patterns of hepatocellular carcinoma in HCV infection. Dig Dis Sci. 1996;14:49S–55S.
Poynard T, Ratziu V, Charlotte F, Goodman Z, McHutchison J, Albrecht J. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis C. J Hepatol. 2001;34:730–739.
Yano M, Kumada H, Kage M, et al. A long term pathological evolution of chronic hepatitis C. Hepatology. 1996;23:1334–1340.
Lok AS, Seeff LB, Morgan TR, et al and the HALT-C Trial Group. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138–148.
Di Bisceglie AM. Hepatitis C and hepatocellular carcinoma. Hepatology. 1997;26:34S–38S.
Chang Y, Lairson DR, Chan W, Lu S-N, Aoki N. Cost effectiveness of screening for hepatocellular carcinoma among subjects at different levels of risk. J Eval Clin Pract. 2011;17:261–267.
Ryder SC. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (HCC) in adults. Gut. 2003;52:iii1–iii8.
Llovet M, Ducreux M. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943.
Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236.
Foster GR, Goldin RD, Main J, Murray-Lyon I, Hargreaves S, Thomas HC. Management of chronic hepatitis C: clinical audit of biopsy based management algorithm. BMJ. 1997;315:453–458.
Sweeting MJ, De Angelis D, Neal KR, Ramsay ME, Irving WL, Wright M. Estimated progression rates in three United Kingdom hepatitis C cohorts differed according to method of recruitment. J Clin Epidemiol. 2006;59:144–152.
Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–2618.
West J, Card T. Reduced mortality rates following elective percutaneous liver biopsies. Gastroenterology. 2010;139:1230–1237.
Echosens. Registered technology of EchoSens. EchoSens, Paris. Available at: www.echosens.com.
Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Clin Hepatol. 2008;28:835–847.
Castera L, Sebastiani G, Le Bail B, de Ledinghen V, Couzigou P, Alberti A. Prospective comparison of two algorithms combining non-invasive methods for staging liver fibrosis in chronic hepatitis C. J Hepatol. 2010;52:191–198.
Myers RP, Pomier-Layrargues G, Kirsch R, et al. Feasibility and diagnostic performance of the FibroScan XL probe for liver stiffness measurement in overweight and obese patients. Hepatology. 2012;55:199–208.
Friedrich-Rust M, Ong MF, Martens S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960–974.
Stebbing J, Farouk L, Panos G, et al. A meta-analysis of transient elastography for the detection of hepatic fibrosis. J Clin Gastroebterol. 2010;44:214–219.
Tzochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas BR, Davidson BR, Burroughs AK. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol. 2011;54:650–659.
Degos F, Perez P, Roche B, et al. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicentre study (the FIBROSTIC study). J Hepatol. 2010;53:1013–1021.
Castera L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, fibrotest, APRI and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350.
Liu S, Schwarzinger M, Carrat F, Goldhaber-Fiebert JD. Cost effectiveness of fibrosis assessment prior to treatment for chronic hepatitis C patients. PLoS ONE. 2011;6:e26783.
Poordad F, McCone J, Bacon BR, et al for the SPRINT-2 investigators. Boceprevir for untreated chronic HCV Genotype 1 infection. N Engl J Med. 2011;364:1195–1206.
Bacon BR, Gordon SC, Lawitz E, et al for the HCV RESPOND-2 Investigators. Boceprevir for previously treated chronic HCV Genotype 1 infection. N Engl J Med. 2011;364:1207–1217.
Wells CD, Murrill WB, Arguedas MR. Comparison of health-related quality of life preferences between physicians and cirrhotic patients: implications for cost-utility analysis in chronic liver disease. Dig Dis Sci. 2004;49:453–458.
Lin OS, Keeffe EB, Sanders GD, Owens DK. Cost-effectiveness of screening for hepatocellular carcinoma in patients with cirrhosis due to chronic hepatitis C. Aliment Pharmacol Ther. 2004;19:1159–1172.
Thompson Coon J, Rogers G, Hewson P, et al. Surveillance of cirrhosis for hepatocellular carcinoma: systematic review and economic analysis. Health Technol Assess. 2007;11:1–206.
Stamuli E, Kruger J, Hutton J. Cost-effectiveness of ultrasound elastography in the assessment of liver fibrosis. Economic report for the NHS Centre for Evidence-based Purchasing. 2009;CEP08053.
Hartwell D, Jones J, Baxter L, Shepherd J. Peginterferon alfa and ribavirin for chronic hepatitis C in patients eligible for shortened treatment, re-treatment or in HCV/HIV co-infection: a systematic review and economic evaluation. Health Technol Assess. 2011;15.
Department of Health. 2010-11 Reference costs publication. Available at: www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_131140. Accessed July 2012.
Treasury HM. The Green Book. Appraisal and Evaluation in Central Government. Treasury Guidance. London: TSO; 2003 (amended 2011).
Stout NK, Knudsen AB, Kong CY, McMahon P, Gazelle S. Calibration methods used in cancer simulation models and suggested guidelines. Pharmacoeconomics. 2009;27:533–545.
Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB. Model transparency and validation: a report of the IPSOR-SMDM modelling good practice task force 7. Med Decis Mak. 2012;32:733–743.
Shepherd J, Jones J, Hartwell D, Davidson P, Price A, Waugh N. Interferon alfa (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess. 2007;11:11.
Tong MJ, el-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion associated hepatitis C. N Engl J Med. 1995;332:1463–1466.
Niederau C, Lange S, Heintges T, et al. Prognosis of chronic hepatitis C: results of a large, prospective cohort study. Hepatology. 1998;28:1687–1695.
Andersson KL, Solomon JA, Goldie SJ, Chung RT. Cost effectiveness of alternative surveillance strategies for hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2008;6:1418–1424.
Abu Dayyeh BK, Yang M, Fuchs BC, et al for the HALT-C Trial Group. A functional polymorphism in the Epydermal growth factor gene is associated with risk of hepatocellular carcinoma. Gastroenterology. 2011;141:141–149.
Donadon V, Balbi M, Mas MD, Casarin P, Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetics with chronic liver disease. Liver Int. 2010;30:750–758.
Pacanowski M, Amur S, Zinek I. New genetic discoveries and treatment for Hepatitis C. JAMA. 2012;307:1921–1922.
Han C, Michalopoulos GK, Wu T. Prostaglandin E2 receptor EP1 transactivates EGFR/MET receptor tyrosine kinases and enhances invasiveness in human hepatocellular carcinoma cells. J Cancer Physiol. 2006;207:261–270.
Marquardt JU, Galle PR, Teufel A. Molecular diagnosis and therapy of hepatocellular carcinoma: an emerging field for advanced technologies. J Hepatol. 2012;56:267–275.
Adam R, Cailliez V, Majno P, et al. Normalised intrinsic mortality risk in liver transplantation: European liver transplant registry study. Lancet. 2000;356:621–627.
Canadian Agency for Drugs and Technologies in Health. Transient elastography (FibroScan) for non-invasive assessment of liver fibrosis. 2012
Fleming KM, Aithal GP, Card TR, West J. All-cause mortality in people with cirrhosis compared with the general population: a population-based cohort study. Liver Int. 2012;32:79–84.
Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318.
Greten TF, Papendorf F, Bleck JS, et al. Survival rate in patients with hepatocellular carcinoma: a retrospective analysis of 389 patients. Br J Cancer. 2005;92:1862–1868.
Livraghi T, Giorgio A, Marin G, et al. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology. 1995;197:101–108.
Mondazzi L, Bottelli R, Brambilla G, et al. Transarterial oily chemoembolization for the treatment of hepatocellular carcinoma: a multivariate analysis of prognostic factors. Hepatology. 1994;19:1115–1123.
Naugler WE, Sonnenberg A. Survival and cost-effectiveness analysis of competing strategies in the management of small hepatocellular carcinoma. Liver Transpl. 2010;16:1186–1194.
Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ. Empirically calibrated model of hepatitis C virus infection in the United States. Am J Epidemiol. 2002:156:761–773.
Trevisani F, Santi V, Gramenzi A, et al for Italian Liver Cancer Group. Surveillance for early diagnosis of hepatocellular carcinoma: is it effective in intermediate/advanced cirrhosis? Am J Gastroenterology. 2007;102:2448–2457.
Connock M, Round J, Bayliss S, Tubeuf S, Greenheld W, Moore D. Sorafenib for the treatment of advanced hepatocellular carcinoma. Health Technol Assess. 2010;14:17–21.
The Global Burden of Hepatitis C Working Group. Global burden of disease (GBD) for hepatitis C. J Clin Pharmacol. 2004;44:20–29.
Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328.
Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–700.
Llovet JM, Mas X, Aponte JJ, et al. Cost effectiveness of adjuvant therapy for hepatocellular carcinoma during the waiting list for liver transplantation. Gut. 2002;50:123–128.
Bismuth H, Majno PE, Adam R. Liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 1999;19:311–322.
Ladabaum U, Cheng SL, Yao FY, Roberts JP. Cost effectiveness of screening for recurrent hepatocellular carcinoma after liver transplantation. Clin Transplant. 2011;25:283–291.
Carr B, Carroll S, Muszbek N, Gondek K. Economic evaluation of Sorafenib in unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 2010;25:1739–1746.
Acknowledgments
CC is funded by MRC Population Health Scientist Fellowship RA0837.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Canavan, C., Eisenburg, J., Meng, L. et al. Ultrasound Elastography for Fibrosis Surveillance Is Cost Effective in Patients with Chronic Hepatitis C Virus in the UK. Dig Dis Sci 58, 2691–2704 (2013). https://doi.org/10.1007/s10620-013-2705-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-013-2705-y