Abstract
Background
Fecal Occult Blood Test (FOBT) is an accepted screening test for colorectal cancer (CRC). It has been shown to decrease mortality by up to 30 %. The outcome of screening failures has not been adequately studied.
Aims
The purpose of this study was to assess the outcome of patients who were diagnosed with CRC after a false negative FOBT.
Methods
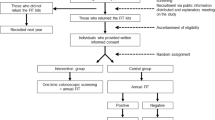
We identified all consecutive CRCs from pathology reports between 2005 and 2010. Patients were divided according to their FOBT result. Those who became positive were compared to patients who remained negative.
Results
Altogether 401 CRCs were identified. Of those, 202 never performed a FOBT. At least one negative FOBT was performed by 133 individuals (67 %). Of these, 76 remained negative (false negatives, FN) and 57 became positive (positive conversion, PC, controls). The prevalence of metastatic disease was threefold higher among the FNs as compared to the PC group (16 [22.2 %] vs. 4 [7.5 %], P = 0.022). All-cause mortality was also significantly higher among FNs versus PCs (24 [31.6 %] vs. 5 [8.8 %], P = 0.001); in Cox regression analysis of survival (covariates: FNs vs. PC, gender, age, medications and co-morbidities) FNs had increased mortality compared to the PC (HR 2.929, P = 0.033, CI 95 % 1.092–7.858). No statistically significant difference was found regarding all primary end points when comparing the FN and the “No test” group.
Conclusion
These data disclose a particular risk of FOBT as a screening test. A subgroup of patients with “false” negative tests may have increased morbidity and mortality. Efforts should be made to recognize and characterize this high-risk group.


Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. Cancer J Clin. 2011;61(2):69–90.
Ahlquist DA, McGill DB, Fleming JL, et al. Patterns of occult bleeding in asymptomatic colorectal cancer. Cancer. 1989;63(9):1826–1830.
Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. N Eng J Med. 1993;328:1365–1371.
Hardcastle JD, Chamberlain JO, Robinson MHE, et al. Randomized controlled trial of fecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472–1477.
Kronborg O, Fenger C, Olsen J, Jørgensen OD, Søndergaard O. Randomised study of screening for colorectal cancer with fecal occult blood test. Lancet. 1996;348:1467–1471.
Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomized controlled trial. Lancet. 2010;375(9726):1624–1633.
Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000;343(22):1603–1607.
Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–1595.
Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale—update based on new evidence. Gastroenterology. 2003;124:544–560.
Lieberman D. Progress and challenges in colorectal cancer screening and surveillance. Gastroenterology. 2010;138:2115–2126.
Van Rossum LG, van Rijn AF, Laheij RJ, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology. 2008;135:82–90.
Hol L, van Leerdam ME, van Ballegooijen M, et al. Screening for colorectal cancer: randomized trial comparing guaiac-based and immunochemical faecal occult blood testing and flexible sigmoidoscopy. Gut. 2010;59:62–68.
Barchana M, Keinan Boker L. An update on colorectal cancer morbidity in Israel; 2011. http://www.old.health.gov.il/Download/pages/crc2011_4.pdf.
Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (Hemoccult): an update. Am J Gastroenterol. 2008;103(6):1541–1549.
Ahlquist DA. Faecal occult blood testing for colorectal cancer: can we afford to do this? Gastroenterol Clin North Am. 1997;26:41–55.
Brasso K, Ladelund S, Frederiksen BL, Jørgensen T. Psychological distress following fecal occult blood test in colorectal cancer screening—a population-based study. Scand J Gastroenterol. 2010;45:1211–1216.
Schnell T, Aranha GV, Sontag SJ, et al. Fecal occult blood testing: a false sense of security? Surgery. 1994;116(4):798–802.
Robinson MH, Hardcastle JD, Moss SM, et al. The risks of screening: data from the Nottingham randomized controlled trial of fecal occult blood screening for colorectal cancer. Gut. 1999;45(4):588–592.
Steele RJ, McClements P, Watling C, et al. Interval cancers in a FOBT-based colorectal cancer population screening program: implications for stage, gender and tumor site. Gut. 2012;61(4):576–581.
Acknowledgments
Financial support for this study was provided by the Israeli Cancer Association.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Elizabeth E. Half and Liat Mlynarsky contributed equally to this work.
Rights and permissions
About this article
Cite this article
Half, E.E., Mlynarsky, L., Naftali, T. et al. False Negative Fecal Occult Blood Test May Be Associated with Increased Mortality from Colorectal Cancer. Dig Dis Sci 58, 2639–2645 (2013). https://doi.org/10.1007/s10620-013-2702-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-013-2702-1