Abstract
Background
Intestinal absorptive capacity shows a circadian rhythm synchronized with eating patterns. Disrupting these coordinated rhythms, e.g., with shift work, may contribute to metabolic disease. Circadian expression of nutrient transporters has not been studied in metabolic disease. We studied the circadian rhythm of intestinal transporter sodium glucose co-transporter type 1 (SGLT1) in an obese diabetic rat.
Methods
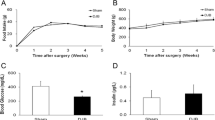
We compared obese Zucker diabetic fatty (ZDF) rats to lean ZDF littermates. Temporal feeding patterns were assessed, then rats were harvested at Zeitgeber (ZT, ZT0 = 7:00 a.m.) 3, 9, or 15 to measure insulin resistance, SGLT1 expression and intestinal glucose absorption capacity. Regulators of SGLT1 (sweet taste receptor T1R2/3; clock genes) were measured to elucidate underlying mechanisms.
Results
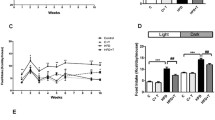
Both groups exhibited altered circadian food intake. Obese ZDF rats lost circadian rhythmicity of SGLT1 mRNA expression and functional activity. Lean ZDF rats maintained rhythmicity of SGLT1 mRNA expression but that of functional glucose absorption was blunted. Circadian rhythms of intestinal clock genes were maintained in both groups. Neither group had discernible rhythms of intestinal GLUT2 (glucose transporter) or T1R2 (sweet taste receptor component) mRNA expression. In summary, lean and obese ZDF rats exhibited similar disruptions in circadian feeding. Glucose intolerance was evident in lean rats, but only obese rats further developed diabetes and exhibited disrupted circadian rhythmicity of both SGLT1 mRNA expression and function.
Conclusions
Our findings suggest that disrupted circadian feeding rhythms contribute to glucose intolerance, but additional factors (genetics, changes in nutrient sensing/transport) are needed to lead to full diabetes.





Similar content being viewed by others
References
Pan A, Schernhammer ES, Sun Q, Hu FB. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med. 2011;8:e1001141.
Biggi N, Consonni D, Galluzzo V, Sogliani M, Costa G. Metabolic syndrome in permanent night workers. Chronobiol Int. 2008;25:443–454.
Culpepper L. The social and economic burden of shift-work disorder. J Fam Pract. 2010;59:S3–S11.
De Bacquer D, Van Risseghem M, Clays E, Kittel F, De Backer G, Braeckman L. Rotating shift work and the metabolic syndrome: a prospective study. Int J Epidemiol. 2009;38:848–854.
Esquirol Y, Bongard V, Mabile L, Jonnier B, Soulat JM, Perret B. Shift work and metabolic syndrome: respective impacts of job strain, physical activity, and dietary rhythms. Chronobiol Int. 2009;26:544–559.
Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity. 2009;17:2100–2102.
Arble DM, Vitaterna MH, Turek FW. Rhythmic leptin is required for weight gain from circadian desynchronized feeding in the mouse. PLoS ONE. 2011;6:e25079.
Mathews CE, Wickwire K, Flatt WP, Berdanier CD. Attenuation of circadian rhythms of food intake and respiration in aging diabetes-prone BHE/Cdb rats. Am J Physiol. Regul Integr Comp Physiol. 2000;279:R230–R238.
Murakami DM, Horwitz BA, Fuller CA. Circadian rhythms of temperature and activity in obese and lean Zucker rats. Am J Physiol. 1995;269:R1038–R1043.
Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453–4458.
Boden G, Chen X, Polansky M. Disruption of circadian insulin secretion is associated with reduced glucose uptake in first-degree relatives of patients with type 2 diabetes. Diabetes. 1999;48:2182–2188.
Corpe CP, Burant CF. Hexose transporter expression in rat small intestine: effect of diet on diurnal variations. Am J Physiol. 1996;271:G211–G216.
Houghton SG, Iqbal CW, Duenes JA, Fatima J, Kasparek MS, Sarr MG. Coordinated, diurnal hexose transporter expression in rat small bowel: implications for small bowel resection. Surgery. 2008;143:79–93.
Balakrishnan A, Stearns AT, Rounds J, et al. Diurnal rhythmicity in glucose uptake is mediated by temporal periodicity in the expression of the sodium-glucose cotransporter (SGLT1). Surgery. 2008;143:813–818.
Tavakkolizadeh A, Berger UV, Shen KR, et al. Diurnal rhythmicity in intestinal SGLT-1 function, V(max), and mRNA expression topography. Am J Physiol Gastrointest Liver Physiol. 2001;280:G209–G215.
Dyer J, Wood IS, Palejwala A, Ellis A, Shirazi-Beechey SP. Expression of monosaccharide transporters in intestine of diabetic humans. Am J Physiol Gastrointest Liver Physiol. 2002;282:G241–G248.
Balakrishnan A, Stearns AT, Ashley SW, Tavakkolizadeh A, Rhoads DB. Restricted feeding phase shifts clock gene and sodium glucose cotransporter 1 (SGLT1) expression in rats. J Nutr. 2010;140:908–914.
Stearns AT, Balakrishnan A, Rhoads DB, Tavakkolizadeh A. Rapid upregulation of sodium-glucose transporter SGLT1 in response to intestinal sweet taste stimulation. Ann Surg. 2010;251:865–871.
Fu WJ, Haynes TE, Kohli R, et al. Dietary l-arginine supplementation reduces fat mass in Zucker diabetic fatty rats. J Nutr. 2005;135:714–721.
Karasov WH, Pond RS 3rd, Solberg DH, Diamond JM. Regulation of proline and glucose transport in mouse intestine by dietary substrate levels. Proc Natl Acad Sci USA. 1983;80:7674–7677.
Ferraris RP, Vinnakota RR. Intestinal nutrient transport in genetically obese mice. Am J Clin Nutr. 1995;62:540–546.
Bihler I, Freund N. Sugar transport in the small intestine of obese hyperglycemic, fed and fasted mice. Diabetologia. 1975;11:387–393.
Morton AP, Hanson PJ. Monosaccharide transport by the small intestine of lean and genetically obese (ob/ob) mice. Q J Exp Physiol. 1984;69:117–126.
Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:1–13.
Balakrishnan A, Stearns AT, Ashley SW, Rhoads DB, Tavakkolizadeh A. PER1 modulates SGLT1 transcription in vitro independent of E-box status. Dig Dis Sci. 2012;57:1525–1536.
Kudo T, Akiyama M, Kuriyama K, Sudo M, Moriya T, Shibata S. Night-time restricted feeding normalises clock genes and Pai-1 gene expression in the db/db mouse liver. Diabetologia. 2004;47:1425–1436.
Tavakkolizadeh A, Ramsanahie A, Levitsky LL, et al. Differential role of vagus nerve in maintaining diurnal gene expression rhythms in the proximal small intestine. J Surg Res. 2005;129:73–78.
Ramsanahie AP, Berger UV, Zinner MJ, Whang EE, Rhoads DB, Ashley SW. Effect of glucagon-like peptide-2 (GLP-2) on diurnal SGLT1 expression. Dig Dis Sci. 2004;49:1731–1737.
Kellett GL, Brot-Laroche E. Apical GLUT2: a major pathway of intestinal sugar absorption. Diabetes. 2005;54:3056–3062.
Ait-Omar A, Monteiro-Sepulveda M, Poitou C, et al. GLUT2 accumulation in enterocyte apical and intracellular membranes: a study in morbidly obese human subjects and ob/ob and high fat-fed mice. Diabetes. 2011;60:2598–2607.
Gorboulev V, Schurmann A, Vallon V, et al. Na(+)-d-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes. 2012;61:187–196.
Kellett GL. Comment on: Gorboulev et al. Na+-d-glucose cotransporter SGLT1 Is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 2012;61:187–196. Diabetes 2012; 61: e4; author reply e5.
Stearns AT, Balakrishnan A, Rhoads DB, Ashley SW, Tavakkolizadeh A. Diurnal expression of the rat intestinal sodium-glucose cotransporter 1 (SGLT1) is independent of local luminal factors. Surgery. 2009;145:294–302.
Young RL, Sutherland K, Pezos N, et al. Expression of taste molecules in the upper gastrointestinal tract in humans with and without type 2 diabetes. Gut. 2009;58:337–346.
Acknowledgments
We thank Jan Rounds for invaluable managerial support, and Dr. Carel Le Roux at Imperial College, London, UK for his role as co-educational supervisor to HB. The manuscript has been presented as an oral presentation at the Society of Academic and Research Surgery, Nottingham UK (Jan 2012) and the Academic Surgical Congress, Las Vegas (Feb 2012). It was published in abstract form only in a supplementary issue of Journal of Surgical Research as related to this meeting. This study was funded by National Institute of Health Grant 1 R01 DK084064 (AT) and Harvard Clinical and Translational Science Center 5 KL2 RR025757 (AT).
Conflict of interest
HY Bhutta, TE Deelman, SW Ashley, and DB Rhoads have no conflicts of interest. A Tavakkoli has an equity interest in Avaxia Biologics, a company that is developing oral antibodies for treatment of intestinal disorders, with potential applications for treatment of diabetes and obesity. AT’s interests were reviewed and are managed by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict-of-interest policies.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10620_2013_2669_MOESM1_ESM.pptx
Diurnal expression of T1R2 (A) and GLUT2 (B) in obese (left) and lean (right) ZDF rats. Values are expressed as medians ± interquartile range, n = 8–11 per group per time point (PPTX 69 kb)
Rights and permissions
About this article
Cite this article
Bhutta, H.Y., Deelman, T.E., Ashley, S.W. et al. Disrupted Circadian Rhythmicity of the Intestinal Glucose Transporter SGLT1 in Zucker Diabetic Fatty Rats. Dig Dis Sci 58, 1537–1545 (2013). https://doi.org/10.1007/s10620-013-2669-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-013-2669-y