Abstract
Background
Osteopontin (OPN) is associated with the Th1 immune response in inflammatory bowel diseases (IBD). While OPN has been shown to play an important role in maintaining the epithelial barrier, its role in IBD remains unclear.
Aim
The aim of this study was to assess OPN function in patients with IBD and in the mouse colitis model.
Methods
Osteopontin expression in colonic samples from IBD patients was determined by a semi-quantitative immunohistochemical staining method. Colitis in BALB/c mice was induced by 5 % dextran sodium sulfate (DSS), followed by treatment with salazosulfapyridine (SASP) and infliximab, respectively. The plasma OPN concentration was measured by an enzyme-linked immunosorbent assay. The expression of OPN in colonic tissues was detected by reverse transcriptase PCR, real-time PCR and Western blot, and the localization of OPN was determined by a semi-quantitative immunohistochemical staining method. The immune function of OPN was investigated by measuring the production of cytokines, and the amount of cytokines produced was then used to determine OPN immune functions.
Results
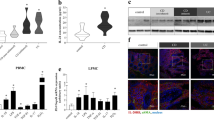
Osteopontin expression in intestinal epithelial cells was significantly lower in IBD patients than in controls, while its expression in lamina propria exudative cells was significantly higher in IBD patients than in controls. In DSS-induced mice, OPN expression in plasma and colonic tissues increased significantly, and this increase was significantly reduced after the mice were treated with SASP and infliximab. OPN promoted the Th1 immune response and strengthened inflammation in the mouse colitis model.
Conclusions
Our results indicate that OPN plays an important role in the immune response and is also involved in the mucosal protective mechanism in IBD.








Similar content being viewed by others
Abbreviations
- CD:
-
Crohn’s disease
- DSS:
-
Dextran sodium sulfate
- IBD:
-
Inflammatory bowel disease
- IECs:
-
Intestinal epithelial cells
- IFN:
-
Interferon
- IL:
-
Interleukin
- OPN:
-
Osteopontin
- TNF:
-
Tumor necrosis factor
- UC:
-
Ulcerative colitis
References
Denhardt DT, Noda M, O’Regan AW, Pavlin D, Berman JS. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 2001;107:1055–1061.
Senger DR, Asch BB, Smith BD, Perruzzi CA, Dvorak HF. A secreted phosphoprotein marker for neoplastic transformation of both epithelial and broblastic cells. Nature. 1983;302:714–715.
Patarca R, Freeman GJ, Singh RP, et al. Structural and functional studies of the early T lymphocyte activation 1 (Eta-1) gene. Definition of a novel T-cell-dependent response associated with genetic resistance to bacterial infection. J Exp Med. 1989;170:145–161.
Ashkar S, Weber GF, Panoutsakopoulou V, et al. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–864.
Chabas D, Baranzini SE, Mitchell D, et al. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science. 2001;294:1731–1735.
Sato T, Nakai T, Tamura N, et al. Osteopontin/Eta-1 upregulated in Crohn’s disease regulates the Th1 immune response. Gut. 2005;54:1254–1262.
Mishima R, Takeshima F, Sawai T, et al. High plasma osteopontin levels in patients with inflammatory bowel disease. J Clin Gastroenterol. 2007;41:167–172.
Gassler N, Autschbach F, Gauer S, et al. Expression of osteopontin (Eta-1) in Crohn disease of the terminal ileum. Scand J Gastroenterol. 2002;37:1286–1295.
Zhong J, Eckhardt ER, Oz HS, Bruemmer D, de Villiers WJ. Osteopontin deficiency protects mice from dextran sodium sulfate-induced colitis. Inflamm Bowel Dis. 2006;12:790–796.
Zohar R, Suzuki N, Suzuki K, et al. Intracellular osteopontin is an integral component of the CD44-ERM complex involved in cell migration. J Cell Physiol. 2000;184:118–130.
Zhu B, Suzuki K, Goldberg HA, et al. Osteopontin modulates CD44-dependent chemotaxis of peritoneal macrophages through G-protein-coupled receptors: evidence of a role for an intracellular form of osteopontin. J Cell Physiol. 2004;198:155–167.
Brown LF, Berse B, van de Water L, et al. Expression and distribution of osteopontin in human tissues: widespread association with luminal epithelial surfaces. Mol Biol Cell. 1992;3:1169–1180.
Qu-Hong, Dvorak AM. Ultrastructural localization of osteopontin immunoreactivity in phagolysosomes and secretory granules of cells in human intestine. Histochem J. 1997;29:801–812.
Da Silva AP, Pollett A, Rittling SR, Denhardt DT, Sodek J, Zohar R. Exacerbated tissue destruction in DSS-induced acute colitis of OPN-null mice is associated with downregulation of TNF-a expression and non-programmed cell death. J Cell Physiol. 2006;208:629–639.
Inflammatory Bowel Disease Co-operation Group. Chinese Society of Gastroenterology. Chinese consensus on diagnosis and treatment standard of inflammatory bowel disease. Chin J Gastroenterol. 2007;12:488–495.
Cooper HS, Murthy SN, Shan RS, Sedergran DJ. Clinicopathogogic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249.
Zhang H, Zhang Y. The quantitative analysis on immunohistochemical image of non-small cell lung cancer biology characteristic. Chin J Stereol Image Anal. 2006;11:77–79.
Masuda H, Takahashi Y, Asai S, Hemmi A, Takayama T. Osteopontin expression in ulcerative colitis is distinctly different from that in Crohn’s disease and diverticulitis. J Gastroenterol. 2005;40:409–413.
Chen S, Kaifang Z, Tian Y, Tan Y, Ding Y, Qian W. Expression and significance of toll-like receptor (TLR) 2, TLR4 and TLR9 in colonic mucosa of rat model with induced colitis. Chin J Gastroenterol. 2007;12:339–343.
Bruemmer D, Collins AR, Noh G, et al. Angiotensin II-accelerated atherosclerosis and aneurysm formation is attenuated in osteopontin-deficient mice. J Clin Invest. 2003;112:1318–1331.
Lakatos L. Immunology of inflammatory bowel diseases. Acta Physiol Hung. 2000;87:355–372.
Sawa Y, Oshitani N, Adachi K, Higuchi K, Matsumoto T, Arakawa T. Comprehensive analysis of intestinal cytokine messenger RNA profile by real-time quantitative polymerase chain reaction in patients with inflammatory bowel disease. Int J Mol Med. 2003;11:175–179.
Dieleman LA, Palmen MJ, Akol H, et al. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immuol. 1998;114:385–391.
Naik S, Kelly EJ, Meijer L, Pettersson S, Sanderson IR. Absence of Toll-like receptor 4 explains endotoxin hyporesponsiveness in human intestinal epithelium. J Pediatr Gastroenterol Nutr. 2001;32:449–453.
Melgar S, Yeung MM, Bas A, et al. Over expression of interleukin 10 in mucosal T cells of patients with active ulcerative colitis. Clin Exp Immunol. 2003;134:127–137.
Xinsheng Yao. Survey and progress of Th1 and Th2. Int J Immunol. 2002;25:290–293.
Lindsay JO, Ciesielski CJ, Scheinin T, Brennan FM, Hodgson HJ. Local delivery of adenoviral vectors encoding murine interleukin 10 induces colonic interleukin 10 production and is therapeutic for murine colitis. Gut. 2003;52:363–369.
Heilmann K, Hoffmann U, Witte E, et al. Osteopontin as two-sided mediator of intestinal inflammation. J Cell Mol Med. 2009;13:1162–1174.
Lund SA, Giachelli CM, Scatena M. The role of osteopontin in inflammatory processes. J Cell Commun Signal. 2009;3:311–322.
Acknowledgments
This work was kindly supported by Grant 09L-38 from Fudan University Young Scholars Foundation (Shanghai, China) and Grant 2011MW01 from the Health Department of Minhang District, Shanghai, China.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, F., Liu, H., Shen, Q. et al. Osteopontin: Participation in Inflammation or Mucosal Protection in Inflammatory Bowel Diseases?. Dig Dis Sci 58, 1569–1580 (2013). https://doi.org/10.1007/s10620-012-2556-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-012-2556-y