Abstract
Background
Lactobacillus consumption has been shown to attenuate the severity of experimental colitis. Whether the effects of Lactobacillus on colitis are related to modulation of leukocyte recruitment into the inflamed intestine is unclear.
Aims
To investigate the effect of Lactobacillus plantarum daily intragastric administration on lymphocyte homing and intestinal inflammation in interleukin 10 (IL-10) knockout mice, an experimental model of colitis.
Methods
Two groups of ten IL-10 knockout mice were fed phosphate buffered saline containing Lactobacillus plantarum 1258 or unmodified vehicle for 4 weeks. Two groups of ten wild-type mice were used as controls. At killing, the bowels were histologically scored and evaluated by transmission electron microscopy. Mucosal addressin cell adhesion molecule 1 (MAdCAM-1) and intercellular adhesion molecule 1 (ICAM-1) expression were determined by immunohistochemistry. The Levels of proinflammatory cytokines, tumor necrosis factor α (TNF-α), and interferon γ (IFN-γ) were determined by ELISA. In addition, levels of CD3, α4β7, ICAM-1, and MAdCAM-1 were determined by reverse-transcription polymerase chain reaction and Western blot.
Results
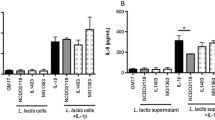
L. plantarum treatment improved the histological damage score in KO mice compared to untreated KO mice. L. plantarum significantly attenuated the expression of MAdCAM-1, ICAM-1, CD3, and α4β7, but did not affect the levels of TNF-α and IFN-γ when treated KO mice were compared to untreated KO mice.
Conclusions
L. plantarum interfered with the upregulation of adhesion molecules observed in IL-10 knockout mice compared to wild-type mice, attenuating the symptoms of colitis.






Similar content being viewed by others
References
Eksteen B, Liaskou E, Adams DH. Lymphocyte homing and its role in the pathogenesis of IBD. Inflamm Bowel Dis. 2008;14(9):1298–1312.
Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347(6):417–429.
Marteau P, Lepage P, Mangin I, et al. Review article: gut flora and inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20(Suppl 4):18–23.
Favier C, Neut C, Mizon C, Cortot A, Colombel JF, Mizon J. Fecal beta-d-galactosidase production and Bifidobacteria are decreased in Crohn’s disease. Dig Dis Sci. 1997;42(4):817–822.
Cummings JH, Macfarlane GT, Macfarlane S. Intestinal bacteria and ulcerative colitis. Curr Issues Intest Microbiol. 2003;4(1):9–20.
Martinez-Medina M, Aldeguer X, Gonzalez-Huix F, Acero D, Garcia-Gil LJ. Abnormal microbiota composition in the ileocolonic mucosa of Crohn’s disease patients as revealed by polymerase chain reaction-denaturing gradient gel electrophoresis. Inflamm Bowel Dis. 2006;12:1136–1145.
Sokol H, Seksik P, Rigottier-Gois L, et al. Specificities of the fecal microbiota in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:106–111.
Tamboli CP, Neut C, Desreumaux P, Colombel JF. Dysbiosis in inflammatory bowel disease. Gut. 2004;53:1–4.
Conte MP, Schippa S, Zamboni I, et al. Gut-associated bacterial microbiota in paediatric patients with inflammatory bowel disease. Gut. 2006;55:1760–1767.
Pagnini C, Cominelli F. Probiotics in experimental and human inflammatory bowel disease: discussion points. Dig Liver Dis. 2006;38(Suppl 2):S270–S273.
Gionchetti P, Rizzello F, Venturi A, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305–309.
Hilton E, Kolakowski P, Smith M, et al. Efficacy of Lactobacillus GG as a diarrheal preventative in travelers. J Travel Med. 1997;4:41–43.
Saavedra J. Probiotics and infectious diarrhea. Am J Gastroenterol. 2000;95:S16–S18.
Canducci F, Armuzzi A, Cremonini F, et al. A lyophilized and inactivated culture of Lactobacillus acidophilus increases Helicobacter pylori eradication rates. Aliment Pharmacol Ther. 2000;14:1625–1629.
Fuller R. A review: probiotics in man and animals. J Appl Bacteriol. 1989;66:365–378.
Guarner F, Schaafsma GJ. Probiotics. Int J Food Microbiol. 1998;39:237–238.
Metchnikoff E. The Prolongation of Life. Optimistic studies. London: William Heinemann; 1907.
Dunne C, O’Mahony L, Murphy L, et al. In vitro selection criteria for probiotic bacteria of human origin: correlation with in vivo findings. Am J Clin Nutr. 2001;73(2 Suppl):386S–392S.
Vanderpool C, Yan F, Polk DB. Mechanisms of probiotic action: implications for therapeutic applications in inflammatory bowel diseases. Inflamm Bowel Dis. 2008;14(11):1585–1596.
Sheil B, Shanahan F, O’Mahony L. Probiotic effects on inflammatory bowel disease. J Nutr. 2007;137:819S–824S.
Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–1633.
Angulo S, Llopis M, Antolín M, et al. Lactobacillus casei prevents the upregulation of ICAM-1 expression and leukocyte recruitment in experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2006;291(6):G1155–G1162.
Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66.
Salmi M, Jalkanen S. Lymphocyte homing to the gut: attraction, adhesion, and commitment. Immunol Rev. 2005;206:100–113.
Sackstein R. The lymphocyte homing receptors: gatekeepers of the multistep paradigm. Curr Opin Hematol. 2005;12:444–450.
Mora JR, von Andrian UH. T-cell homing specificity and plasticity: new concepts and future challenges. Trends Immunol. 2006;27:235–243.
Marelli-Berg FM, Cannella L, Dazzi F, Mirenda V. The highway code of T cell trafficking. J Pathol. 2008;214:179–189.
Marelli-Berg FM, Okkenhaug K, Mirenda V. A two-signal model for T cell trafficking. Trends Immunol. 2007;28:267–273.
Krüger K, Mooren FC. T cell homing and exercise. Exerc Immunol Rev. 2007;13:37–54.
Madsen K, Cornish A, Soper P, et al. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121(3):580–591.
Schultz M, Veltkamp C, Dieleman LA, et al. Lactobacillus plantarum 299 V in the treatment and prevention of spontaneous colitis in interleukin-10-deficient mice. Inflamm Bowel Dis. 2002;8:71–80.
Saverymuttu SH, Camilleri M, Rees H, Lavender JP, Hodgson HJ, Chadwick VS. Indium 111-granulocyte scanning in the assessment of disease extent and disease activity in inflammatory bowel disease. A comparison with colonoscopy, histology, and fecal indium 111-granulocyte excretion. Gastroenterology. 1986;90:1121–1128.
Souza HS, Elia CC, Spencer J, MacDonald TT. Expression of lymphocyte-endothelial receptor-ligand pairs, alpha4beta7/MAdCAM-1 and OX40/OX40 ligand in the colon and jejunum of patients with inflammatory bowel disease. Gut. 1999;45(6):856–863.
Bento AF, Leite DF, Claudino RF, Hara DB, Leal PC, Calixto JB. The selective nonpeptide CXCR2 antagonist SB225002 ameliorates acute experimental colitis in mice. J Leukoc Biol. 2008;84(4):1213–1221.
Feighery LM, Smith P, O’Mahony L, Fallon PG, Brayden DJ. Effects of Lactobacillus salivarius 433118 on intestinal inflammation, immunity status and in vitro colon function in two mouse models of inflammatory bowel disease. Dig Dis Sci. 2008;53(9):2495–2506.
Madsen KL, Doyle JS, Jewell LD, Tavernini MM, Fedorak RN. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology. 1999;116(5):1107–1114.
McCarthy J, O’Mahony L, O’Callaghan L, et al. Double-blind, placebo-controlled trial of two probiotic strains in interleukin-10 knockout mice and mechanistic link with cytokine balance. Gut. 2003;52(7):975–980.
Gupta P, Andrew H, Kirschner BS, Guandalini S. Is Lactobacillus GG helpful in children with Crohn’s disease? Results of a preliminary, open-label study. J Pediatr Gastroenterol Nutr. 2000;31(4):453–457.
Miele E, Pascarella F, Giannetti E, Quaglietta L, Baldassano RN, Staiano A. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am J Gastroenterol. 2009;104(2):437–443.
van Assche G, Rutgeerts P. Antiadhesion molecule therapy in inflammatory bowel disease. Inflamm Bowel Dis. 2002;8(4):291–300.
Okada Y, Tsuzuki Y, Miyazaki J, et al. Propionibacterium freudenreichii component 1,4-dihydroxy-2-naphthoic acid (DHNA) attenuates dextran sodium sulphate induced colitis by modulation of bacterial flora and lymphocyte homing. Gut. 2006;55(5):681–688.
Nakamura M, Saito H, Kasanuki J, et al. Cytokine production in patients with inflammatory bowel disease. Gut. 1992;33:933–937.
Ando T, Jordan P, Wang Y, et al. MAdCAM-1 expression and regulation in murine colonic endothelial cells in vitro. Inflamm Bowel Dis. 2005;11:258–264.
Pathmakanthan S, Li CK, Cowie J, Hawkey CJ. Lactobacillus plantarum 299: beneficial in vitro immunomodulation in cells extracted from inflamed human colon. J Gastroenterol Hepatol. 2004;19(2):166–173.
Peña JA, Rogers AB, Ge Z, et al. Probiotic Lactobacillus spp. diminish Helicobacter hepaticus-induced inflammatory bowel disease in interleukin-10-deficient mice. Infect Immun. 2005;73(2):912–920.
Acknowledgments
This work was supported by the National Key Basic Research Plan Program of China (973 Program), No. 2008CB517403, and the Special Foundation for Academician Jie-Shou Li (Intestinal Barrier Research), No. LJS_2009001.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chu, ZX., Chen, HQ., Ma, YL. et al. Lactobacillus plantarum Prevents the Upregulation of Adhesion Molecule Expression in an Experimental Colitis Model. Dig Dis Sci 55, 2505–2513 (2010). https://doi.org/10.1007/s10620-009-1063-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-009-1063-2