Abstract
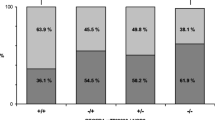
Dopamine and its receptors may be involved in inflammatory reaction. The availability of this molecule depends on its receptors. The DRD2 gene, which codifies for the D2 dopamine receptor, has several polymorphisms. In this study, the DRD2 TaqIA polymorphism, which confers a decreased receptor density, was evaluated in 313 individuals including 220 inflammatory bowel disease patients (143 patients with Crohn’s disease and 77 with ulcerative colitis) and in 93 healthy blood donors. The analysis was carried out by PCR-RFLP techniques. The frequencies of A 1 A 1 and A 2 A 2 genotypes were similar among Crohn’s disease, ulcerative colitis patients, and health controls. Also, the genotype frequency was similar in different groups of disease localization, behavior, and age of disease onset. However, the Crohn’s disease patients carriers of A 2 A 2 genotype showed a lower risk for development refractory Crohn’s disease (37 out 65) than A 1 A 1 and A 1 A 2 carriers (28 out of 65) [(OR=0.4, 95% CI 0.21–0.87; p=0.02)]. Our results support an involvement of the dopamine receptor in inflammatory bowel disease and suggest a new potential target for therapy in refractory Crohn’s disease patients.
Similar content being viewed by others
References
Groux H, Powrie F (1999) Regulatory T cells and inflammatory bowel disease. Immunol Today 20:442–445
Strober W, Kelsall B, Fuss I, Marth T, Ludviksson B, Ehrhardt R, Neurath M (1997) Reciprocal IFN-gamma and TGF-beta responses regulate the occurrence of mucosal inflammation. Immunol Today 18:61–64
Stangi J, Parkes M, Louis E (1996) Two stage genoma-with search in inflammatory bowel disease provides evidence for susceptibility loci on chromossoma 3,7 and 12. Nat Genet 14:199–202
Hugot JP, Chamaillard M, Zouali H (2001) Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 411:599–603
Ogura Y, Bonen DK, Inohara N (2001) A frameshift mutation in NOD2 associated with susceptibility to Cohn’s disease. Nature 411:603–606
Ader S, Felton D, Cohn N (1990) Interactions between the brain and the immune system. Annu Rev Pharmacol Toxicol 30:561–602
Basu S, Dasgupta PS (2000) Dopamine, a neurotransmitter, influences the immune system. J Neuroimmunol 102:113–124
McKenna F, Mclaughlin PJ, Lweis BJ, Sibbring GC, Cumerson JA, Bowen-Jones D (2002) Dopamine receptor expression on human T and B-lymphocytes, monocytes, neutrophils, eosinophils and NK cells: a flow cytometric study. J Neuroimmunol 132:34–40
Ricci A, Vegilio F, Amenta F (1995) Radioligand binding characterization of putative dopamine D3 receptor in human peripheral blood lymphocytes with 3H-7-OH-DPAT. J Neuroimmunol 58:139–144
Bergquist J, Tarkowski A, Ekman R, Ewing A (1994) Discovery of endogenous catecholamines in lymphocytes and evidence for catecholamines regulation of lymphocytes function via an autocrine loop. Proc Natl Acad Sci USA 91:12912–12916
Magro F, Vieira-Coelho MA, Fraga S, Serrao MP, Veloso FT, Ribeiro T, Soares-da-Silva P (2002) Impaired synthesis or cellular storage of norepinephrine, dopamine, and 5-hydroxytryptamine in human inflammatory bowel disease. Dig Dis Sci 47:216–224
Magro F, Fraga S, Ribeiro T, Soares-da-Silva P (2004) Decreased availability of intestinal dopamine in transmural colitis may relate to inhibitory effects of interferon-gamma upon L-dopa uptake. Acta Physiol Scand 180:379–386
Harvey RF, Bradshaw JM (1980) A simple index of Crohn’s disease activity. Lancet 1(8167):514
Irvine EJ (1995) Usual therapy improves Crohn’s disease as measure a new disease activity index. McMaster Study Group. J Clin Gastroenterol 20:27–32
Truelove SC, Witts LJ (1995) Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J 4947:1041–1048
Munkholm P, Langholz E, Davidsen M, Binder V (1994) Frequency of glucocorticoid resistance and dependency in Crohn’s disease. Gut 35:360–362
Potocnik U, Ferkolj I, Glavac D, Dean M (2004) Polymorphisms in multidrug resistance 1 (MDR1) gene are associated with refractory Crohn’s disease and ulcerative colitis. Genes and Immunity 5:530–539
Spitz MR, Shi H, Yang F, Hudmon KS, Jiang H, Chamberlain RM, Amos CI, Wan Y, Cinciripini P, Hong WK, Wu X (1998) Case-control study of the D2 dopamine receptor gene and smoking status in lung cancer patients. J Natl Cancer Inst 90:358–363
Niessner M, Volk BA (1995) Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reverse transcribed polymerase chain reaction (RT-PCR). Clin Exp Immunol 101:428–435
Breese E, Braegger CP, Corrigan CJ (1993). Interleukin-2- and interferon-gamma-secreting T cells in normal and diseased human intestinal mucosa. Immunol Today 78:127–131
Papadakis KA, Targan SR (2000) Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med 51:289–298
Peppelenbosch MP, Deventer SJ (2004) T cell apoptosis and inflammatory bowel disease. Gut 53:1556–1558
Ghosh MC, Mondal AC, Basu S, Banerjee S, Majumber J, Bhattacharya D, Dasgupta PA (2003) Dopamine inhibits cytokine release and expression of tyrosine kinases, LCK and Fyn in activated T cells. Int Immunopharmacol 3:1019–1026
Denny MF, Patai B, Strauss DB (2000) Differential T-cell antigen receptor signaling mediated by Src family kinases Lck and Fyn. Mol Cell Biol 20:1426–1435
Qian D, Weiss A (1997) T cell antigen receptor signal transduction. Curr Opin Cell Biol 9:205–212
Morikawa K, Oseko F, Morikawa S (1994) Immunosuppressive activity of bromocriptine on human T lymphocyte function in vitro. Clin Exp Immunol 95:514–518
Riskind PN, Massacesi L, Doolittle TH, Hauser SL (1991) The role of prolactin in autoimmune demyelination: suppression of experimental allergic encepalomyelitis by bromocriptine. Ann Neurol 29:542–547
Bergquist J, Josefsson E, Tarkowski A, Ekman R, Ewing A (1997) Measurements of catecholamine-mediated apoptosis of immunocompetent cells by capillary electrophoresis. Electrophoresis 18:1760–1766
Colombo C, Cosentino M, Marino F, Rasini E, Ossola M, Blandini F, Mangiagalli A, Samuele A, Ferrari M, Bombelli R, Lecchini S, Nappi G, Frigo G (2003) Dopaminergic modulation of apoptosis in human blood mononuclear cells: possible relevance of Parkinson’s disease. Ann N Y Acad Sci 1010:679–682
Figueroa FE, Carrion F, Martinnez ME, Rivero S, Mamani L (1997) Bromocriptine induces immunological changes related to disease parameters in rheumatoid arthritis. Br J Rheumatol 36:1022–1023
McMurray RW, Weidensaul D, Allen SH, Walker SE (1995) Efficacy of bromocriptine in an open label therapeutic trial for systemic lupus erythematosus. J Reumatol 22:2084–2091
Weber G, Frey H (1987) Treatment of psoriatic arthritis with bromocriptine. J Am Acad Dermatol 16:388–389
Kast RE, Altschuler EL (2001) Remission of Crohn’s disease on Bupropion. Gastroenterol 121:1260–1261
Bergquist J, Silberring J (1998) Identification of catecholamines in the immune system by electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom 12:683–688
Josefsson E, Bergquist J, Ekman R, Tarkowski A (1996) Catecholamines are synthesized by mouse lymphocytes and regulate function of these cells by induction of apoptosis. Immunology 88:140–146
Tsao CW, Lin YS, Cheng JT (1998) Inhibition of immune cell proliferation with haloporinol and relationship of tyrosine hydroxylase expression to immune cell growth. Life Sci 62:PL335–PL344
Ricci A, Bronzetti E, Felici L, Greco S, Amenta F (1998) Labeling of dopamine D3 and D4 receptor subtypes in human peripheral blood lymphocytes with 3H7-OH-DPAT: a combined radioligand binding assay and immunochemical study. J Neuroimmunol 92:191–195
Ricci A, Bronzetti E, Mignini F, Tayebati SK, Zaccheo D, Amenta F (1999) Dopamine d1-like receptor subtypes in human peripheral blood lymphocytes. J Neuroimmunol 96:234–240
Levite M, Chowers Y, Ganor Y, Besser M, Hershkovits R, Cahalon L (2001) Dopamine interacts directly with its D3 and D2 receptors on normal human T cells, and activates beta 1 integrin function. Eur J Immunol 31:3504–3512
Civelli O, Buzow JR, Grandy DK (1993) Molecular diversity of dopamine receptors. Annu Rev Pharmacol Toxicol 32:281–307
Gingrich JA, Caron MG (1993) Recent advances in molecular biology of dopamine receptors. Annu Rev Neurosci 16:299–321
Grandy DK, Litt M, Allen L, Bunzow JR, Marchionni M, Makam H, Reed L, Magenis RE, Civelli O (1989) The human D(2) dopamine receptor gene is located on chromosome 11 at q22-q23 and identifies a TaqI RFLP. Am J Hum Genet 45:778–785
Noble EP, Blum K, Ritchie T, Montgomery A, Sheridan PJ (1991) Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch Gen Psych 48:648–654
Noble EP, St Jeor ST, Ritchie T, Syndulko K, St Jeor SC, Fitch RJ, Brunner RL, Sparkes RS (1994) D2 dopamine receptor gene and cigarette smoking: a reward gene? Med Hypotheses 42:257–260
Epstein LH, Wright SM, Paluch RA, Leddy JJ, Hawk LWJ, Jaroni JL, Saad FG, Crystal-Mansour S, Shields PG, Lerman C (2004) Relation between food reinforcement and dopamine genotypes and its effect on food intake in smokers. Am J Clin Nutr 80:82–88
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Magro, F., Cunha, E., Araujo, F. et al. Dopamine D2 Receptor Polymorphisms in Inflammatory Bowel Disease and the Refractory Response to Treatment. Dig Dis Sci 51, 2039–2044 (2006). https://doi.org/10.1007/s10620-006-9168-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-006-9168-3