Abstract
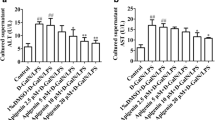
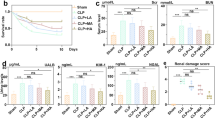
Nafamostat mesilate (NM) is a synthetic protease inhibitor with various biological effects. To determine its effect on liver injury related to sepsis, we investigated the effects of NM on lipopolysaccharide (LPS)-induced liver injury. Wistar rats were allocated into two groups; the NM group underwent intraperitoneal NM administration 30 min before LPS administration, and the control group underwent PBS administration. Serum AST and ALT levels were significantly decreased in NM-treated rats. Reduced levels of TNF-α, IL-1β, and IFN-γ were observed after LPS administration in NM-treated rats. No significant differences were observed in IL-6 levels between the NM and the control group. In contrast, HGF levels were significantly increased only in control rats. NM treatment decreased protein and mRNA levels of TLR-4 and CD14. Our data suggest that NM treatment has protective effects against LPS-induced hepatotoxicity through downregulation of TLR4 and CD14 in liver, which decreased TNF-α, IL-1β, and IFN-γproduction in liver.





Similar content being viewed by others
References
Raetz CR, Ulevitch RJ, Wright SD, Sibley CH, Ding A, Nathan CF (1991) Gram-negative endotoxin: an extraordinary lipid with profound effects on eukaryotic signal transduction. FASEB J 5:2652–2560
Suzuki S, Nakamura S, Serizawa A, Sakaguchi T, Konno H, Muro H (1996) Role of Kupffer cells and the spleen in modulation of endotoxin-induced liver injury after partial hepatectomy. Hepatology 24:219–225
Enomoto N, Ikejima K, Bradford B, Rivera C, Kono H, Brenner DA (1998) Alcohol causes both tolerance and sensitization of rat Kupffer cells via mechanisms dependent on endotoxin. Gastroenterology 115:443–451
Whalan C, Drew P, Maddern G (1998) Infection, sepsis and systemic inflammatory response syndrome in obstructive jaundice. J Gastroenterol Hepatol 13:354–355
Nolan JP (1989) Intestinal endotoxins as mediators of hepatic injury—an idea whose time has come again. Hepatology 10:887–891
Thiele DL (1989) Tumor necrosis factor, the acute phase response and the pathogenesis of alcohol liver disease. Hepatology 9:497–499
Guarner F, Wallage JL, MacNaughton WK (1989) Endotoxin-induced ascitis formation in the rat: partial mediation by platelet activating factor. Hepatology 10:788–794
Okajima K, Uchiba M, Murakami K (1995) Nafamostat mesilate. Cardiovasc Drug Rev 13:51–65
Paques EP, Romisch J (1991) Comparative study on the in vitro effectiveness of antithrombotic agents. Thromb Res 64:11–21
Uchiba M, Okajima K, Abe H, Okabe H, Takatsuki K (1994) Effect of nafamostat mesilate, a synthetic protease inhibitor, on tissue factor-factor VIIa complex activity. Thromb Res 74:155–161
Takahashi H, Takizawa S, Tatewaki W, Nagai K, Wada K, Hanano M, Shibata A (1989) Nafamostat mesilate (FUT-175) in the treatment of patients with disseminated intravascular coagulation. Thromb Haemost 62:372
Yoshikawa T, Murakami M, Furukawa Y, Kato H, Takemura S, Kondo M (1983) Effects of FUT–175, a new synthetic protease inhibitor on endotoxin-induced disseminated intravascular coagulation in rats. Hemostasis 13:374–378
Kikuchi M, Endo S, Inada K, Yamashita H, Takakuwa T, Nakae H, Kasai T, Baba N, Yamada Y (1995) Inhibitory effect of FUT–175 on the production of interleukin-8 and polymorphonuclear leukocyte elastase. Res Commun Mol Pathol Pharm 87:269–274
Sugita H, Ishiko T, Ikei S, Hirota M, Ogawa M (1999) FUT-175 inhibits the production of IL-6 and IL-8 in human monocytes. Res Commun Mol Pathol Pharm 103:57–64
Ikehara S, Shimamura K, Aoyama T (1985) Effects of FUT–175, a new synthetic protease inhibitor, on the development of lupus nephritis in (NZBxNZW)F1 mice. Immunology 55:494–500
Turner A, Keyhani AH, Reiner R (1981) Proteolytic enzymes released by macrophages may promote hepatic injuries in the rat model of hepatic damage. Gastroenterology 80:647–654
Turner A, Keyhani AH, Wright R (1983) The influence of endotoxin in vitro on hepatic macrophage lysosomal enzyme release in different rat model of hepatic injury. Liver 3:151–160
O'Neill LA, Dinarello CA (2000) The IL-1 receptor/toll-like receptor superfamily: crucial receptors for inflammation and host defense. Immunol Today 21:206–209
Aderem A, Ulevitch RJ (2000) Toll-like receptors in the induction of the innate immune response. Nature 408:740–745
Hemmi H, Takeuchi O, Kawai T, Kaisho S, Sato H, Sanjo H (2000) A Toll-like receptor recognizes bacterial DNA. Nature 408:740–745
Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F (1999) Toll-like receptor-5 mediates lipopolysaccharide-induced signal transduction. J Biol Chem 274:10689–10692
Rush BF Jr, Sori AJ, Murphy TF, Smith S, Flanagan JJ Jr, Machiedo GW (1998) Endotoxemia and bacteremia during hemorrhagic shock. The link between trauma and sepsis? Ann Surg 207:549–554
Sedman PC, Macfie J, Sagar P, Mitchell CJ, May J, Mancey-Jones B (1994) The prevalence of gut translocation in humans. Gastroenterology 107:643–649
Wells CL, Maddaus MA, Simmons RL (1988) Proposed mechanism for the translocation of intestinal bacteria. Rev Infect Dis 10:958–979
Grotz MR, Deitch EA, Ding J, Xu D, Huang Q, Regel G (1999) Intestinal cytokine response after gut ischemia: role of gut barrier failure. Ann Surg 229:478–486
Pain JA, Bailey MEE (1987) Measurement of operative plasma endotoxin levels in jaundiced and non-jaundiced patients. Eur Surg Res 19:207–216
Van Bossuyt H, Desmaretz C, Gaeta GB, Wisse E (1990) The role of bile acids in the development of endotoxemia during obstructive jaundice in the rat. J Hepatol 10:274–279
Nanji AA, Khettry U, Sadrzadeh SM (1994) Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver disease. Proc Soc Exp Biol Med 205:243–247
Choda Y, Morimoto Y, Miyaso H, Shinoura S, Saito S, Yagi T, Iwagaki H, Tanaka N (2004) Failure of the gut barrier system enhances liver injury in rats—Protection of hepatocytes by gut-derived hepatocyte growth factor. Eur J Hepatogastroenterol (in press)
Ogawa M, Mori Y, Ueda S, Mori T, Makino Y, Hori J, Ohto M, Wakashin M (1993) Protective effects of FUT-175 on acute massive hepatic necrosis induced in mice following endotoxin injection and immunization with liver proteins. J Hepatol 19:393–400
Inagaki H, Nonami T, Kurokawa T, Takeuchi Y, Okuda N, Nakao A, Sakamoto J (1999) Effects of nafamostat mesilate, a synthetic protease inhibitor, on immunity and coagulation after hepatic resection. Hepato-Gastroenterology 46:3223–3228
Davie EW, Fujikawa K, Kisiel W (1991) The coagulation cascade: initiation, maintenance and regulation. Biochemistry 30:10365–10370
Kaplan JE, Malik AB (1987) Thrombin-induced intravascular coagulation: role in vascular injury. Semin. Thromb Hemost 13:398–415
Hasegawa N, Husari AW, Hart WT, Kandra TG, Raffin TA (1995) Role of the coagulation system in ARDS. Chest 105:268–277
Uchiba M, Okajima K, Murakami K, Okabe H, Takatsuki K (1995) Endotoxin-induced pulmonary vascular injury is mainly mediated by activated neutrophils in rats. Thromb Res 78:117–125
Brett J, Gerlach H, Nawroth P, Steinberg S, Godman G, Stern D (1989) Tumor necrosis factor/cachectin increases permeability of endothelial cell monolayers by a mechanism involving regulatory G proteins. J Exp Med 169:1977–1991
Stephens KE, Ishizaka A, Wu ZH, Larrick JW, Raffin TA (1988) Granulocyte depletion prevents tumor necrosis factor-mediated acute lung injury in guinea pigs. Am Rev Respir Dis 138:1300–1307
Abe H, Okajima K, Okabe H, Takatsuki K, Binder BR (1994) Granulocyte proteases and hydrogen peroxide synergistically inactivate thrombomodulin of endothelial cells in vitro. J Lab Clin Med 123:874–881
Fujioka N, Mukaida N, Harada A, Akiyama M, Kasahara T, Kuno K (1995) Preparation of specific antibodies against murine IL-1ra and the establishment of IL-1ra as an endogenous regulator of bacteria-induced fulminant hepatitis in mice. J Leuko Biol 58:90–98
Okamura H, Tsutsui H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T (1995) Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 378:88–91
Nicoletti F, Di Marco R, Zaccone P, Salvaggio A, Magro G, Bendtzen K (2000) Murine concanavalin A-induced hepatitis is prevented by interleukin-12 (IL-12) antibody and exacerbated by exogenous IL-12 through an interferon-gamma-dependent mechanism. Hepatology 32:728–733
Solomon KR, Kurt-Jones EA, Saladino RA, Stack AM, Dunn IF, Ferretti M (1998) Heterotrimeric G proteins physically associated with the lipopolysaccharide receptor CD14 modulate both in vivo and in vitro responses to lipopolysaccharide. J Clin Invest 102:2019–2027
Medzhitov R, Preston-Hurlburt P, Janeway CA Jr (1997) A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388:394–397
Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F (1999) Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem 274:10689–10692
Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P (1999) Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J Exp Med 189:615–625
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Miyaso, H., Morimoto, Y., Ozaki, M. et al. Protective Effects of Nafamostat Mesilate on Liver Injury Induced by Lipopolysaccharide in Rats: Possible Involvement of CD14 and TLR-4 Downregulation on Kupffer Cells . Dig Dis Sci 51, 2007–2012 (2006). https://doi.org/10.1007/s10620-006-9141-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-006-9141-1