Abstract
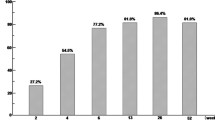
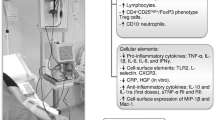
Depletion of granulocytes and monocytes (GM) by selective apheresis (GMA) with an Adacolumn exerts an anti-inflammatory effect in patients with ulcerative colitis (UC) or rheumatoid arthritis. However, the mechanism of the anti-inflammatory effect of GMA is not fully understood yet. We investigated the effect of GMA on the plasma concentration of interleukin-1 receptor antagonist (IL-1ra), a potent anti-inflammatory cytokine. Twenty-six patients with active UC received GMA at one session per week for 5 consecutive weeks. Clinical response was defined as Δclinical activity index (ΔCAI=CAI at entry – CAI at post)≥4, while clinical remission was defined as CAI≤4. Twenty-one of twenty-six patients (80.8%) responded to GMA. In the first session, plasma from responder patients showed a significant (P < 0.01) increase in IL-1ra in the Adacolumn outflow. In contrast, there was no change in IL-1ra in nonresponders. In conclusion, release of IL-1ra during GMA might be one mechanism of clinical efficacy associated with this therapy.



Similar content being viewed by others
References
Saniabadi AR, Hanai H, Suzuki Y, Ohmori T, Sawada K, Yoshimura N, Saito Y, Takeda Y, Umemura K, Kondo K, Ikeda Y, Fukunaga K, Nakashima M, Beretta A, Bjarnason I, Lofberg R (2005) Adacolumn for selective leukocytapheresis as a non-pharmacological treatment for patients with disorders of the immune system: An adjunct or an alternative to drug therapy? J Clin Apher 20:171–184
Saniabadi AR, Hanai H, Takeuchi K, Bjarnason I, Lofberg R (2003) Adacolumn, an adsorptive carrier based granulocyte and monocyte apheresis device for the treatment of inflammatory and refractory diseases associated with leukocytes. Ther Apher Dial 7:48–59
Ohara M, Saniabadi AR, Kokuma S, Hirata I, Adachi M, Agishi T, Kasukawa R (1997) Granulocytapheresis in the treatment of patients with rheumatoid arthritis. Artif Organs 21:989–994
Kashiwagi N, Sugimura K, Koiwai H, Yamamoto H, Yoshikawa T, Saniabadi AR, Adachi M, Shimoyama T (2002) Immunomodulatory effects of granulocyte and monocyte adsorption apheresis as a treatment for patients with ulcerative colitis. Dig Dis Sci 47:1334–1341
Braddock M, Quinn A (2004) Targeting IL-1 in inflammatory disease: new opportunities for therapeutic intervention. Nat Rev Drug Discov 3:330–339
Rogler G, Andus T (1998) Cytokines in inflammatory bowel disease. World J Surg 22:382–389
Eisenberg SP, Evans RJ, Arend WP, Verderber E, Brewer MT, Hannum CH, Thompson RC (1990) Primary structure and functional expression from complementary DNA of a human interleukin-1 receptor antagonist. Nature 343:341–346
Hannum CH, Wilcox CJ, Arend WP, Joslin FG, Dripps DJ, Heimdal PL, Armes LG, Sommer A, Eisenberg SP, Thompson RC (1990) Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature 343:336–340
Arend WP (2002) The mode of action of cytokine inhibitors. J Rheumatol Suppl 65:16–21
Mansfield JC, Holden H, Tarlow JK, Di Giovine FS, McDowell TL, Wilson AG, Holdsworth CD, Duff GW (1994) Novel genetic association between ulcerative colitis and the anti-inflammatory cytokine interleukin-1 receptor antagonist. Gastroenterology 106:637–642
Andus T, Daig R, Vogl D, Aschenbrenner E, Lock G, Hollerbach S, Kollinger M, Scholmerich J, Gross V (1997) Imbalance of the interleukin 1 system in colonic mucosa—-association with intestinal inflammation and interleukin 1 receptor antagonist [corrected] genotype 2. Gut 41:651–657
Vijgen L, Van Gysel M, Rector A, Thoelen I, Esters N, Ceelen T, Vangoidsenhoven E, Vermeire S, Rutgeerts P, Van Ranst M (2002) Interleukin-1 receptor antagonist VNTR-polymorphism in inflammatory bowel disease. Genes Immun 3:400–406
Horai R, Saijo S, Tanioka H, Nakae S, Sudo K, Okahara A, Ikuse T, Asano M, Iwakura Y (2000) Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J Exp Med 191:313–320
Nicklin MJ, Hughes DE, Barton JL, Ure JM, Duff GW (2000) Arterial inflammation in mice lacking the interleukin 1 receptor antagonist gene. J Exp Med 191:303–312
Arend WP (1993) Interleukin-1 receptor antagonist. Adv Immunol 54:167–227
Malyak M, Smith MFJr, Abel AA, Arend WP (1994) Peripheral blood neutrophil production of interleukin-1 receptor antagonist and interleukin-1 beta. J Clin Immunol 14:20–30
Kyogoku M, Kasukawa R (1998) Clinical and basic studies on the G-1 column, a new extracorporeal therapeutic device effective in controlling rheumatoid arthritis. Inflamm Res 47(Suppl 3):166–176
Hiraishi K, Takeda Y, Shiobara N, Shibusawa H, Jimma F, Kashiwagi N, Saniabadi AR, Adachi M (2003) Studies on the mechanisms of leukocyte adhesion to cellulose acetate beads: an in vitro model to assess the efficacy of cellulose acetate carrier-based granulocyte and monocyte adsorptive apheresis. Ther Apher Dial 7:334–340
Hanai H, Watanabe F, Takeuchi K, Saniabadi AR, Bjarnason I (2003) Leukocyte adsorptive apheresis for the treatment of active ulcerative colitis: a prospective, uncontrolled study. Clin Gastroenterol Hepatol 1:28–35
Kashiwagi N, Hirata I, Kasukawa R (1998) A role for granulocyte and monocyte apheresis in the treatment of rheumatoid arthritis. Ther Apher 2:134–141
Hanai H, Watanabe F, Saniabadi AR, Matsushitai I, Takeuchi K, Iida T (2002) Therapeutic efficacy of granulocyte and monocyte adsorption apheresis in severe active ulcerative colitis. Dig Dis Sci 47:2349–2353
Rachmilewitz D (1989) Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ 298:82–86
Matts SG (1961) The value of rectal biopsy in the diagnosis of ulcerative colitis. Q J Med 30:393–407
Shimoyama T, Sawada K, Hiwatashi N, Sawada T, Matsueda K, Munakata A, Asakura H, Tanaka T, Kasukawa R, Kimura K, Suzuki Y, Nagamachi Y, Muto T, Nagawa H, Iizuka B, Baba S, Nasu M, Kataoka T, Kashiwagi N, Saniabadi AR (2001) Safety and efficacy of granulocyte and monocyte adsorption apheresis in patients with active ulcerative colitis: a multicenter study. J Clin Apher 16:1–9
Propst A, Propst T, Herold M, Vogel W, Judmaier G (1995) Interleukin-1 receptor antagonist in differential diagnosis of inflammatory bowel diseases. Eur J Gastroenterol Hepatol 7:1031–1036
Kuboyama S (1998) Increased circulating levels of interleukin-1 receptor antagonist in patients with inflammatory bowel disease. Kurume Med J 45:33–37
Takeda Y, Shiobara N, Saniabadi AR, Adachi M, Hiraishi K (2004) Adhesion dependent release of hepatocyte growth factor and interleukin-1 receptor antagonist from human blood granulocytes and monocytes: Evidence for the involvement of plasma IgG, complement C3 and beta2 integrin. Inflamm Res 53:277–283
Symons JA, Young PR, Duff GW (1995) Soluble type II interleukin 1 (IL-1) receptor binds and blocks processing of IL-1 beta precursor and loses affinity for IL-1 receptor antagonist. Proc Natl Acad Sci USA 92:1714–1718
Burger D, Chicheportiche R, Giri JG, Dayer JM (1995) The inhibitory activity of human interleukin-1 receptor antagonist is enhanced by type II interleukin-1 soluble receptor and hindered by type I interleukin-1 soluble receptor. J Clin Invest 96(1):38–41
Takeda Y, Hiraishi K, Takeda H, Shiobara N, Shibusawa H, Saniabadi AR, Adachi M, Kawata S (2003) Cellulose acetate beads induce release of interleukin-1 receptor antagonist, but not tumour necrosis factor-alpha or interleukin-1beta in human peripheral blood. Inflamm Res 52:287–290
Acknowledgment
The authors thank Mr. N. Shiobara of Japan Immunoresearch Laboratories for his technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sakimura, K., Omori, T., Iwashita, E. et al. Clinical Response Is Associated with Elevated Plasma Interleukin-1 Receptor Antagonist During Selective Granulocyte and Monocyte Apheresis in Patients with Ulcerative Colitis. Dig Dis Sci 51, 1525–1531 (2006). https://doi.org/10.1007/s10620-005-9012-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-005-9012-1