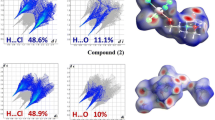
The synthesis of four-armed materials based on a pentaerythritol core, which is connected by spacers with different lengths to the coumarin moieties as mesogenic groups, is reported. The chemical structure of compounds is confirmed by FT-IR, 1H, 13C NMR, and mass spectrometery (MS). UV-Vis spectroscopy measurements exhibited a red shift in peaks of coumarin ring from 318 to 366 nm compared with free coumarin molecules, which is attributed to the developed π–π interactions by possible formation of liquid crystal structures. These materials are interesting regarding their potential application in LCDs.

Similar content being viewed by others
References
Z. Wen, K. Yang, and J. M. Raquez, Molecules, 25, 1241 (2020).
M. Castillo-Valles, A. Martinez-Bueno, R. Gimenez, T. Sierra, and M. B. Ros, J. Mater. Chem. C., 7, 14454 (2019).
D. J. R. Cristaldi, S. Pennisi, and F. Pulvirenti, Liquid Crystal Display Drivers, Techniques and Circuits, First Edition, Springer Science + Business Media B.V., 2009.
K. Mehta, A. R. Peeketi, L. Liu, D. Broer, P. Onck, and R. K. Annabattula, Appl. Phys. Rev., 7, 041306 (2020).
P. Bhagavath, R. Shetty, and D. Sunil, Crit. Rev. Solid State Mater. Sci., 45, 378 (2020).
S. K. Sharma and H. Demir, Green Chemistry in Scientific Literature: A Bibliometric Study and Research Trends, Taylor & Francis Group, NY, 2020.
R. Najjar, E. Bigdeli, K. Asadpour-Zeynali, and M.-S. Zaker-Hamidi, J. Electr. Mater., 47, 7143 (2018).
M. Molnar, M. Loncaric, and M. Kovac, Curr. Org. Chem., 24, 4 (2020).
H. Mostafavi, R. Najjar, and E. Maskani, Chem. Nat. Compd., 49, 423 (2013).
M. Mohammadi Zeydi, S. J. Kalantarian, and Z. Kazeminejad, J. Iran. Chem. Soc., 17, 3031 (2020).
R. Najjar and E. Bigdeli, Eur. Polym. J., 104, 136 (2018).
D. Sh. Yao, B. Y. Zhang, Y. H. Li, and W. Q. Xiao, Tetrahedron Lett., 45, 8953 (2004).
J. Qu, X. Luo, and Z. Wang, J. Polym. Sci., Part A: Polym. Chem., 54, 1969 (2016).
X. Zhang, J. Xia, and K. Matyjaszewski, Macromolecules, 33, 2340 (2000).
J. P. Kennedy and S. Jacob, Acc. Chem. Res., 31, 341 (1998).
K. Ohno, B. Wong, and D. M. Haddleton, J. Polym. Sci., Part A: Polym. Chem., 39, 2206 (2001).
X. Wang, H. Zhang, G. Zhong, and X. Wang, Polym., 45, 3637 (2004).
T. Xin-De, Z. Qi-Zhen, L. Ai-Xian, F. Xing-He, C. Xiao-Fan, and Z. Qi-Feng, Chin. J. Chem., 22, 1034 (2004).
J. Ahmed, J. Wu, S. Mushtaq, and Y. Zhang, Today Commun., 23, 100840 (2020).
C. P. Chen, D. S. Kim, and C. G. Jhun, Crystals, 9, 364 (2019).
J. Guo, J. Zhang, Q. Zhang, N. Jiang, and J. Wei, RSC Adv., 3, 21620 (2013).
R. Najjar, M. Akbari, A. Mirmohseni, and M. G. Hosseini, J. Taiwan Inst. Chem. Eng., 93, 1–10 (2018).
R. Najjar, S. A. Katourani, and M. G. Hosseini, Prog. Org. Coat., 124, 110 (2018).
M. Hoshiyama, K. Kubo, T. Igarashi, and T. Sakurai, J. Photochem. Photobiol., A., 138, 227 (2001).
S. Patil and M. R. Bagewadi, IOSR J. Appl. Chem., 12, 80 (2019).
Y. Pana, P. Cai, M. F. Farahani, Y. Li, X. Houb, and H. Xiao, Appl. Surf. Sci., 385, 333 (2016).
B. Sarkanj, M. Molnar, M. Cacic, and L. Gille, Food Chem., 139, 488 (2013).
S. Zhang, D. Fu, H. Sun, X. Yue, Y. Liu, Y. Zhang, and H. Liu, Chem. Heterocycl. Compd., 52, 374 (2016).
M. Ikawa and K. Link, J. Am. Chem. Soc., 72, 4373 (1950).
T. Yasuda, F. Shimizu Liu, G. Ungar, and T. Kato, J. Am. Chem. Soc., 133, 13437 (2011).
S. Ozgan and M. Okumus, Braz. J. Phys., 41, 118 (2011).
G. Pathak, S. Pandey, R. Katiyar, A. Srivastava, R. Dabrowski, K. Garbat, and R. Manohar, J. Lumin., 192, 33 (2017).
A. Concellon, T. Liang, A. Schenning, J. L. Serrano, P. Romero, and M. Marcos, J. Mater. Chem. C., 6, 1000 (2017).
W. L. F. Armarego, Purification of Laboratory Chemicals, 8th Ed., Elsevier Inc., 2017.
K. Mohammed Khan, Z. S. Saify, S. Begum, F. Noor, M. Z. Khan, S. Hayat, M. I. Choudhary, S. Perveen, A. U. Rahman, and Z. Ullah, Nat. Prod. Res., 17, 115 (2010).
Z. Khademi, K. Nikoofar, and F. Shahriyari, Curr. Org. Synth., 16, 38 (2019).
A. D. Singh, S. Kumar, S. Saini, and H. Prabha, Asian Pac. J. Health Sci., 4, 184 (2017).
L. Pan, D. Lei, L. Jin, Y. He, and Q. V. Yang, Molecules, 23, 3002 (2018).
L. Pan, X.-Z. Li, D.-A. Sun, H. Jin, H.-R. Guo, and B. Qin, Chin. Chem. Lett., 27, 375 (2016).
Acknowledgment
The authors acknowledge the financial support of the Postgraduate Studies Office of the University of Tabriz.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 1, January–February, 2023, pp. 26–30.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Afshanmehr, A., Najjar, R. Synthesis and Characterization of Four-Armed Pentaerythritol- and Coumarin-Based Materials as Potential Liquid Crystals. Chem Nat Compd 59, 26–31 (2023). https://doi.org/10.1007/s10600-023-03910-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-023-03910-2