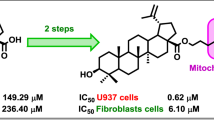
A new method for synthesizing BODIPY-labeled betulinic acid derivatives with a terminal mitochondrialtargeting triphenylphosphonium group on side-chain C-28 was developed. The key synthetic step was cross-coupling between C-2 propynyl derivatives of betulinic acid and halides of fluorescent dye derivatives containing an iodo functional group on C-2 or C-8 of the BODIPY platform. The developed procedure for covalent bonding of the BODIPY fluorophore to betulinic acid enables the native 3-OH and 28-COOH groups to be preserved in the triterpene core.

Similar content being viewed by others
References
F. B. Mullauer, J. H. Kessler, and J. P. Medema, Anti-Cancer Drugs, 21, 215 (2010).
H. Sheng and H. Sun, Nat. Prod. Rep., 28, 543 (2011).
R. Csuk, Expert Opin. Ther. Pat., 24, 913 (2014).
S. Amiri, S. Dastghaib, M. Ahmadi, P. Mehrbod, F. Khadem, H. Behrouj, M. R. Aghanoori, F. Machaj, M. Ghamsari, J. Rosik, A. Hudecki, A. Afkhami, M. Hashemi, M. J. Los, P. Mokarram, T. Madrakian, and S. Ghavami, Biotechnol. Adv., 38, 107409 (2020).
S. Fulda and G. Kroemer, Drug Discovery Today, 14, 885 (2009).
X. Zhang, J. Hu, and Y. Chen, Mol. Med. Rep., 14, 4489 (2016).
X. Wang, X. Lu, R. Zhu, K. Zhang, S. Li, Z. Chen, and L. Li, Neurochem. Res., 42, 1130 (2017).
D. A. Nedopekina, R. R. Gubaidullin, V. N. Odinokov, P. V. Maximchik, B. Zhivotovsky, Y. P. Bel′skii, V. A. Khazanov, A. V. Manuylova, V. Gogvadze, and A. Yu. Spivak, MedChemComm, 8, 1934 (2017).
A. Yu. Spivak, D. A. Nedopekina, R. R. Khalitova, R. R. Gubaidullin, V. N. Odinokov, Y. P. Bel′skii, N. V. Bel′skaya, and V. A. Khazanov, Med. Chem. Res., 26, 518 (2017).
O. V. Tsepaeva, A. V. Nemtarev, T. I. Abdullin, L. R. Grigor′eva, E. V. Kuznetsova, R. A. Akhmadishina, L. E. Ziganshina, H. H. Cong, and V. F. Mironov, J. Nat. Prod., 80, 2232 (2017).
Y. Ye, T. Zhang, H. Yuan, D. Li, H. Lou, and P. Fan, J. Med. Chem., 60, 6353 (2017).
J. Lin, K. Yang, and E. J. Elizabeth, Org. Biomol. Chem., 19, 9339 (2021).
J. Banuelos, Chem. Rec., 16, 335 (2016).
M. R. Martinez-Gonzalez, A. Urias-Benavides, E. Alvarado-Martinez, J. C. Lopez, A. M. Gomez, M. del Rio, I. Garcia, A. Costela, J. Banuelos, T. Arbeloa, I. L. Arbeloa, and E. Pena-Cabrera, Eur. J. Org. Chem., 2014, 5659 (2014).
S. Osati, H. Ali, and J. E. van Lier, J. Porphyrins Phthalocyanines, 20, 61 (2016).
S. Krajcovicova, J. Stankova, P. Dzubak, M. Hajduch, M. Soural, and M. A. Urban, Chem. Eur. J., 24, 4957 (2018).
Z. Li, E. Mintzer, and R. Bittman, J. Org. Chem., 71, 1718 (2006).
B. Brandes, S. Hoenke, L. Fischer, and R. Csuk, Eur. J. Med. Chem., 185, 111858 (2020).
D. Kodr, J. Stankova, M. Rumlova, P. Dzubak, J. Rehulka, T. Zimmermann, I. Krizova, S. Gurska, M. Hajduch, P. B. Drasar, and M. Jurasek, Biomedicines, 9, 1104 (2021).
R. H. Cichewicz and S. A. Kouzi, Med. Res. Rev., 24, 90 (2004).
A. Loudet and K. Burgess, Chem. Rev., 107, 4891 (2007).
Y. Xie, F. Zhang, P. Liu, F. Hao, and H. Luo, Can. J. Chem., 92, 49 (2014).
B. Basumatary, A. Raja Sekhar, R. V. Ramana Reddy, and J. Sankar, Inorg. Chem., 54, 4257 (2015).
M. J. Ortiz, A. R. Agarrabeitia, G. Duran-Sampedro, J. Banuelos Prieto, T. A. Lopez, W. A. Massad, H. A. Montejano, N. A. Garcia, and I. Lopez Arbeloa, Tetrahedron, 68, 1153 (2012).
A. Yu. Spivak, R. R. Gubaidullin, Z. R. Galimshina, D. A. Nedopekina, and V. N. Odinokov, Tetrahedron, 72, 1249 (2016).
A. Yu. Spivak, R. R. Khalitova, R. R. Gubaidullin, and D. A. Nedopekina, Chem. Nat. Compd., 57, 123 (2021).
W. Qin, T. Rohand, W. Dehaen, J. N. Clifford, K. Driesen, D. Beljonne, B. Van Averbeke, M. Van der Auweraer, and N. Boens, J. Phys. Chem. A, 111, 8588 (2007).
V. Leen, T. Leemans, N. Boens, and W. Dehaen, Eur. J. Org. Chem., 2011, 4386 (2011).
D. Prasannan, D. Raghav, S. Sujatha, H. Hareendrakrishna Kumar, K. Rathinasamy, and C. Arunkumar, RSC Adv., 6, 80808 (2016).
A. M. Brouwer, Pure Appl. Chem., 83, 2213 (2011).
Acknowledgment
The work was performed in the framework of scientific research plans of the IPC, UFRC, RAS, State Reg. Nos. FMRS-2022-0081 and FMRS-2022-0074 and with financial support from a Stipend of the President of the Russian Federation for Young Scientists and Graduate Students (SP-317.2022.4). Structural studies of synthesized compounds utilized the Agidel′ Common Use Center at UFRC, RAS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 6, November–December, 2022, pp. 893–899.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Spivak, A.Y., Davletshin, E.V., Gubaidullin, R.R. et al. Synthesis of Bodipy-Labeled Fluorescent Betulinic Acid Derivatives with a Terminal Triphenylphosphonium Group on Side-Chain C-28. Chem Nat Compd 58, 1062–1068 (2022). https://doi.org/10.1007/s10600-022-03869-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-022-03869-6