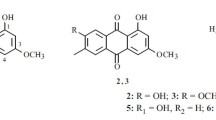
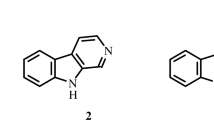
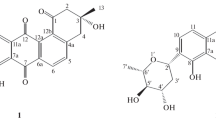
A new macrolide, named concanamycin H (1), together with 12 known compounds (2–13), was isolated from the endophytic Streptomyces humidus SCB0232 for the first time. Compounds 1–13 were identified by analysis of spectroscopic data. Compounds trienomycin A (2) and trienomycin E (3) exhibited moderate inhibitory activities against Fusarium verticillioides and Phytophthora infestans with IC50 values ranging from 32.64 to 72.35 μg·mL–1.

Similar content being viewed by others
References
M. Goodfellow and S. T. Williams, Annu. Rev. Microbiol., 37, 189 (1983).
S. Das, L. R. Ward, and C. Burke, Aquaculture, 305, 32 (2010).
R. Subramani and W. Aalbersberg, Microbiol. Res., 167, 571 (2012).
A. Muller, M. Wenzel, H. Strahl, F. Grein, T. N. V. Saaki, B. Kohl, T. Siersma, J. E. Bandow, H. G. Sahl, T. Schneider, and L. W. Hamoen, Proc. Natl. Acad. Sci. USA, 113, E7077 (2016).
B. Springer, Y. G. Kidan, T. Prammananan, K. Ellrott, E. C. Bottger, and P. Sander, Antimicrob. Agents Chemother., 45, 2877 (2001).
J. Wang, S. M. Soisson, K. Young, W. Shoop, S. Kodali, A. Galgoci, R. Painter, G. Parthasarathy, Y. S. Tang, R. Cummings, S. Ha, K. Dorso, M. Motyl, H. Jayasuriya, J. Ondeyka, K. Herath, C. Zhang, L. Hernandez, J. Allocco, A. Basilio, J. R. Tormo, O. Genilloud, F. Vicente, F. Pelaez, L. Colwell, S. H. Lee, B. Michael, T. Felcetto, C. Gill, L. L. Silver, J. D. Hermes, K. Bartizal, J. Barrett, D. Schmatz, J. W. Becker, D. Cully, and S. B. Singh, Nature, 441, 358 (2006).
J. T. Woo, C. Shinohara, K. Sakai, K. Hasumi, and A. Endo, J. Antibiot., 45, 1108 (1992).
H. Kinashi, K. Someno, and K. Sakaguchi, J. Antibiot., 37, 1333 (1984).
Y. Morimitsu and A. Hirota, Biosci. Biotechnol. Biochem., 60, 1507 (1996).
H. Nomoto, S. Katsumata, K. Takahashi, S. Funayama, K. Komiyama, I. Umezawa, and S. Omura, J. Antibiot., 42, 479 (1989).
H. Inoue, Y. Yajima, G. Kawano, H. Nagasawa, and S. Sakuda, Biocontrol Sci., 9, 39 (2004).
S. W. Yang and G. A. Cordell, J. Nat. Prod., 60, 44 (1997).
Y. Maejima, H. Nakatsugawa, D. Ichida, M. Maejima, Y. Aoyagi, T. Maoka, and H. Etoh, Biosci. Biotechnol. Biochem., 75, 1708 (2011).
P. J. Zhao, H. X. Wang, G. H. Li, H. D. Li, J. Liu, and Y. M. Shen, Chem. Biodiv., 4, 899 (2007).
L. I. Li-Ya, Z. W. Deng, Y. U. Shan-Jiang, and G. U. Jia, Nat. Prod. Res. Dev., 19, 928 (2007).
T. T. Jong and M. Y. Jean, J. Chin. Chem. Soc., 40, 399 (1993).
G. E. Feresin, A. Tapia, M. Sortino, S. Zacchino, A. R. De Arias, A. Inchausti, G. Yaluff, J. Rodriguez, C. Theoduloz, and G. Schmeda-Hirschmann, J. Ethnopharmacol., 88, 241 (2003).
T. Murata, N. Mori, and R. Nishida, J. Chem. Ecol., 37 (10), 1099 (2011).
R. Ottria, S. Casati, and P. Ciuffreda, Magn. Reson. Chem., 50, 823 (2012).
L. Xu, P. Wu, J. Xue, I. Molnar, and X. Wei, J. Nat. Prod., 80, 2215 (2017).
Acknowledgment
This research was financially supported by the National Science Foundation of China (31772032), State Key Laboratory of Applied Microbiology of Southern China (SKLAM006-2015), and Scientific Research Projects of Huizhou University (20170406122437260).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 3, May–June, 2022, pp. 339–341.
Rights and permissions
About this article
Cite this article
Lv, ZC., Zhou, XL., Yan, JH. et al. Secondary Metabolites and Antifungal Activity of the Endophytic Fungus Streptomyces humidus SCB0232 from Water Chestnut. Chem Nat Compd 58, 390–393 (2022). https://doi.org/10.1007/s10600-022-03689-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-022-03689-8