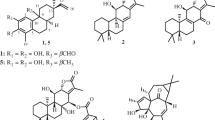
A new stilbenoid, methyl 2,4-dihydroxy-6-(4-hydroxyphenethyl)-3-methylbenzoate (1), along with 11 known compounds, cirsimaritin (2), cirsiliol (3), quercetin (4), apigenin (5), acacetin (6), genkwanin (7), luteolin (8), caffeic acid (9), esculetin (10), alternariol (11), and alternariol-5-O-methyl ether (12), was isolated from the 70% EtOH extract of Caryopteris incana. The structures of these compounds were established via extensive spectroscopic techniques, including 1D, 2D NMR, and HR-ESI-MS. Compound 1 exhibited moderate cytotoxic activity against human HepG2 and SMMC-7721 hepatoma cell lines with IC50 values of 21.3 and 20.1 μM, respectively.

Similar content being viewed by others
References
Y. L. Chen, Flora Reipublicae Popularis Sinicae [in Chinese], Beijing, Science Press, 1982.
S. M. Zhao, G. X. Chou, Q. S. Yang, W. Wang, and J. L. Zhou, Org. Biomol. Chem., 14, 3510 (2016).
C. G. Zhang, T. Chen, X. D. Mao, S. M. Zhao, and G. X. Chou, J. Nat. Med., 73, 210 (2018).
X. D. Mao, G. X. Chou, S. M. Zhao, and C. G. Zhang, Molecules, 21, 1749 (2016).
M. Luczkiewicz, A. Jesionek, A. Kokotkiewicz, P. Migas, M. Mardarowicz, A. Szreniawa-Sztajnert, B. Zabiegala, and A. Bucinski, Acta Physiol. Plantarum, 37, 1 (2015).
S. Park, M. J. Son, C. S. Yook, C. Jin, Y. S. Lee, and H. J. Kim, Phytochemistry, 101, 83 (2014).
X. N. Ji, L. Shi, Y. Wang, and H. Yan, Nat. Prod. Res. Dev., A01, 16 (2014).
J. E. Lee, E. H. Lee, B. O. Kim, and Y. J. Cho, J. Appl. Biol. Chem., 60, 61 (2017).
J. J. Gao, K. Igalashi, and M. Nukina, Biosci. Biotechnol. Biochem., 63, 983 (1999).
K. Yoshikawa, A. Harada, K. Iseki, and T. Hashimoto, Chin. J. Nat. Med., 68, 231 (2014).
M. M. Marzouk, S. R. Hussein, M. E. S. Kassem, S. A. Kawashty, and S. I. M. E, Nat. Prod. Res., 30, 1537 (2016).
C. Frezza, A. Venditti, G. Matrone, I. Serafini, S. Foddai, A. Bianco, and M. Serafini, Nat. Prod. Res., 32, 1583 (2017).
J. D. Guthrie, R. T. Connor, M. F. Stansbury, and T. R. Savich, J. Am. Chem. Soc., 66, 1794 (1944).
C. C. Shen, J. J. Cheng, H. L. Lay, S. Y. Wu, C. L. Ni, C. M. Teng, and C. C. Chen, J. Nat. Prod., 75, 198 (2012).
N. D. Chaurasiya, V. Gogineni, K. M. Elokely, F. Leon, M. J. Nunez, M. L. Klein, L. A. Walker, S. J. Cutler, and B. L. Tekwani, J. Nat. Prod., 79, 2538 (2016).
W. P. Jones, T. Loboecheverri, Q. Mi, H. B. Chai, D. D. Soejarto, G. A. Cordell, S. M. Swanson, and A. D. Kinghorn, J. Nat. Prod., 70, 372 (2007).
M. T. Yasuda, K. Fujita, T. Hosoya, S. Imai, and K. Shimoi, J. Agric. Food Chem., 63, 7693 (2015).
Y. Q. Yin, Z. B. Shen, and L. Y. Kong, J. Chin. Med. Mater., 31, 1501 (2008).
Z. L. Du, Z. Q. Yin, L. Wang, W. C. Ye, W. B. Shen, and S. X. Zhao, Nat. Prod. Res. Dev., 20, 630 (2008).
A. H. Aly, E. Edrada, I. D. Indriani, and M. Wray, J. Nat. Prod., 71, 972 (2008).
F. M. Talontsi, M. T. Islam, P. Facey, C. Douanla-Meli, A. Tidemann, and H. Laatsch, Phytochem. Lett., 5, 657 (2012).
Acknowledgment
This project was financially supported by the National Natural Science Foundation of China (No. 31700303), Hunan Natural Science Foundation (No. 2018JJ3839), the Postgraduates Innovation Program of Central South University (Nos. 1053320184128 and 1053320192764), the Central South University postgraduate independent exploration and innovation Project (Nos. 2019zzts767 and 2020zzts828), and the Open Sharing Fund for Large-scale Instruments and Equipments of Central South University.
Dekun Chen and Kai Zhang contributed equally to this work.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 2, March–April, 2022, pp. 176–178.
Rights and permissions
About this article
Cite this article
Chen, D., Zhang, K., Tan, J. et al. A New Stilbenoid from Caryopteris incana. Chem Nat Compd 58, 199–202 (2022). https://doi.org/10.1007/s10600-022-03640-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-022-03640-x