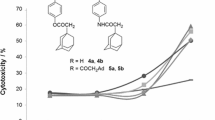
Mono- and bis-piperazinylamides of lupane acids and their derivatives modified on ring A were synthesized and tested for cytotoxicity. Cytotoxicity studies against nine different human tumor cells found that betulonic acid N-methylpiperazinylamide inhibited the growth of SR leukemia, NCI-H460 non-small-cell lung cancer, and HCT-116 colon cancer cells. Cyanoethyl, hydrazone, and oxime moieties in the C3 position had positive effects on the cytotoxicity. The activity spectrum of acanthochlamic diacid amides was broader. The absence/ presence of an N-ethyl group on the piperazine ring affected the cancer type and breadth of cell lines.
Similar content being viewed by others
References
J. A. R. Salvador, A. S. Leal, A. S. Valdeira, B. M. F. Goncalves, D. P. S. Alho, S. A. C. Figueiredo, S. M. Silvestre, and V. I. S. Mendes, Eur. J. Med. Chem., 142, 95 (2017).
U. Bildziukevich, N. Vida, L. Rarova, M. Kolar, D. Saman, L. Havlicek, P. Drasar, and Z. Wimmer, Steroids, 100, 27 (2015).
A. G. Pokrovsky, M. A. Pokrovsky, I. Ya. Mainagashev, N. F. Salakhutdinov, and G. A. Tolstikov, RU Pat. No. 2,445,317, Mar. 20, 2012.
A. G. Pokrovsky, M. A. Pokrovsky, I. Ya. Mainagashev, N. F. Salakhutdinov, and G. A. Tolstikov, RU Pat. No. 2,448,115, Apr. 20, 2012.
E. S. Kurbatov, V. V. Kostruba, V. I. Kazeib, R. N. Karapetyan, and S. V. Kurbatova, Russ. J. Org. Chem., 47, 1267 (2011).
G. V. Giniyatyllina, I. E. Smirnova, O. B. Kazakova, N. P. Yavorskaya, I. S. Golubeva, O. S. Zhukova, R. B. Pugacheva, G. N. Apryshko, and V. V. Poroikov, Med. Chem. Res., 24, 3423 (2015).
G. V. Giniyatullina, O. B. Kazakova, I. P. Baikova, E. Yu. Yamansarov, I. A. Osterman, E. S. Komarova, D. A. Skvortsov, I. V. Saltikova, A. G. Majouga, and Y. A. Ivanenkov, Nat. Prod. Commun., 14, 1 (2019).
E. F. Khusnutdinova, A. V. Petrova, O. B. Kazakova, and A. E. Barmashov, Russ. J. Bioorg. Chem., 45, 552 (2019).
A.-L. Li, Y. Hao, W.-Y. Wang, Q.-S. Liu, Y. Sun, and W. Gu, Int. J. Mol. Sci., 21, 2876 (2020).
B. Brandes, L. Koch, S. Hoenke, H.-P. Deigner, and R. Csuk, Steroids, 163, 108713 (2020).
S. Friedrich, I. Serbian, S. Hoenke, R. K. Wolfram, and R. Csuk, Med. Chem. Res., 29, 906 (2020).
O. B. Kazakova, G. V. Giniyatullina, G. A. Tolstikov, N. I. Medvedeva, T. M. Utkina, and O. L. Kartashova, Russ. J. Bioorg. Chem., 36, 383 (2010).
K.-T. Chue, M.-S. Chang, and L. N. Ten, Chem. Nat. Compd., 47, 759 (2011).
K.-T. Chue, T.-H. Kim, and L. Ten, US Pat. 8,865,935 B2, Oct. 21, 2014.
G. N. Robinson, C. T. Wild, M. Ashton, R. Thomas, C. Montalbetty, T. S. Coulter, F. Magaraci, R. J. Townsend, T. J. Nitz, and J. M. Covert, WO Pat. 2006/053255, May 18, 2006.
K. Qian, I. D. Bori, C.-H. Chen, L. Huang, and K.-H. Lee, J. Med. Chem., 55, 8128 (2012).
S. C. B. Gnoatto, S. Susplugas, L. D. Vechia, T. B. Ferreira, A. Dassonville-Klimpt, K. R. Zimmer, C. Demailly, S. D. Nascimento, J. Guillon, P. Grellier, H. Verli, G. Gosmann, and P. Sonnet, Bioorg. Med. Chem., 16, 771 (2008).
W. Nie, J.-G. Luo, X.-B. Wang, H. Yin, H.-B. Sun, H.-Q. Yao, and L.-Y. Kong, Chem. Pharm. Bull., 59, 1051 (2011).
T. Li, P. Fan, Y. Ye, Q. Luo, and H. Lou, Anti-Cancer Agents Med. Chem., 17, 1153 (2017).
S. Sommerwerk, L. Heller, C. Kerzig, A. E. Kramell, and R. Csuk, Eur. J. Med. Chem., 127, 1 (2017).
P. A. Reddy, P. R. Bandi, M. S. Vedula, R. R. Kura, P. K. Mamnoor, B. R. Kasireddy, N. Mogili, V.- K. Mukkera, V. S. Lanka, and M. R. Ravi, WO Pat. 2013/160810, Oct. 31, 2013.
J. Klinot, V. Sumanova, and A. Vystrcil, Coll. Czech. Chem. Commun., 37, 603 (1972).
G. V. Giniyatullina, O. B. Kazakova, N. I. Medvedeva, I. V. Sorokina, N. A. Zhukova, T. G. Tolstikova, and G. A. Tolstikov, Russ. J. Bioorg. Chem., 39, 329 (2013).
G. A. Tolstikov, L. A. Baltina, V. P. Grankina, R. M. Kondratenko, and T. G. Tolstikova, Licorice: Biodiversity, Chemistry, and Medical Application [in Russian], Geo, Novosibirsk, 2007, 307 pp.
M. C. Alley, D. A. Scudiero, P. A. Monks, M. L. Hursey, M. J. Czerwinski, D. L. Fine, B. J. Abbott, J. G. Mayo, R. H. Shoemaker, and M. R. Boyd, Cancer Res., 48, 589 (1988).
M. R. Grever, S. A. Schepartz, and B. A. Chabner, Semin. Oncol., 19, 622 (1992).
G. V. Giniyatullina, A. G. Mustafin, and O. B. Kazakova, Chem. Nat. Compd., 56, 92 (2020).
O. B. Flekhter, E. I. Boreko, L R. Nigmatullina, E. V. Tret′yakova, N. I. Pavlova, L. A. Baltina, S. N. Nikolaeva, O. V. Savinova, V. F. Eremin, F. Z. Galin, and G. A. Tolstikov, Russ. J. Bioorg. Chem., 30, 80 (2004).
Acknowledgment
The work was performed under topics of State Tasks Nos. AAAA-A19-119020890014-7 and AAAA-A20-120012090023-8. We thank the National Cancer Institute (NCI) for determining the in vitro antitumor activity of 2–5, 7–15, and 17–24.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 4, July–August, 2021, pp. 596–602.
Rights and permissions
About this article
Cite this article
Giniyatullina, G.V., Kazakova, O.B. Synthesis and Cytotoxicity of Lupane Mono- and Bis-Piperazinylamides. Chem Nat Compd 57, 698–705 (2021). https://doi.org/10.1007/s10600-021-03453-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-021-03453-4