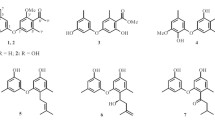
Two new naphthalene derivatives, 1-(3-hydroxy-1-(hydroxymethyl)-2-methoxy-6-methylnaphthalen-7-yl) propan-2-one (1) and 1-(3-hydroxy-1-(hydroxymethyl)-6-methylnaphthalen-7-yl)propan-2-one (2), together three known naphthalene derivatives (3–5) were isolated from the fermentation products of the endophytic fungus Phomopsis fukushii. Their structures were elucidated by spectroscopic methods, including extensive 1D and 2D NMR techniques. Compounds 1 and 2 were evaluated for their anti-methicillin-resistant Staphylococcus aureus (anti-MRSA) activity. The results showed that compounds 1 and 2 showed weak inhibition with IZD of 10.2 ± 1.8 and 11.3 ± 2.0 mm, respectively.
Similar content being viewed by others
References
S. Gupta, P. Chaturvedi, M. G. Kulkarni, and J. Van Staden, Biotechnol. Adv., 39, 107462 (2020).
J. J. Kellogg and H. A. Raja, Phytochem. Rev., 16, 271 (2016).
Y. T. Wang, Y. R. Xue, and C. H. Liu, Mar. Drugs, 13, 4594 (2015).
A. H. Aly, A. Debbab, and P. Proksch, Pharmazie, 68, 499 (2013).
P. L. N. de Carvalho, E. D. Silva, D. A. Chagas-Paula, J. H. H. Luiz, and M. Ikegaki, Mini-Rev. Med. Chem., 16, 259 (2016).
S. K. Deshmukh, S. A. Verekar, and S. V. Bhave, Front. Microbiol., 5, 715 (2015).
S. Kaul, S. Gupta, M. Ahmed, and M. K. Dhar, Phytochem. Rev., 11, 487 (2012).
G. Du, Z. C. Wang, W. Y. Hu, K. L. Yan, X. L. Wang, H. M. Yang, H. Y. Yang, Y. H. Gao, Q. Liu, and Q. F. Hu, Phytochem. Lett., 21, 287 (2017).
X. M. Li, Y. C. Zeng, J. H. Chen, Y. K. Yang, J. Li, L. Ye, G. Du, M. Zhou, Q. F. Hu, G. Y. Yang, H. Y. Yang, and Y. Q. Duan, Chem. Nat. Compd., 55, 618 (2019).
H. Y. Yang, Y. Q. Duan, Y. K. Yang, J. Li, X. Liu, L. Ye, Q. L. Mi, W. S. Kong, M. Zhou, G. Y. Yang, X. M. Li, and Q. F. Hu, Phytochem. Lett., 22, 266 (2017).
Y. H. Gao, R. Zheng, J. Li, W. S. Kong, X. Liu, L. Ye, Q. L. Mi, W. S. Kong, M. Zhou, G. Y. Yang, Q. F. Hu, G. Du, H. Y. Yang, and X. M. Li, J. Asian Nat. Prod. Res., 21, 316 (2019).
Z. J. Li, H. Y. Yang, J. Li, X. Liu, L. Ye, W. S. Kong, S. Y. Tang, G. Du, Z. H. Liu, M. Zhou, G. Y. Yang, Q. F. Hu, and X. M. Li, J. Antibiot., 71, 359 (2018).
S. S. Hu, M. J. Liang, Q. L. Mi, W. Chen, J. Ling, X. Chen, J. Li, G. Y. Yang, Q. F. Hu, W. G. Wang, and Y. D. Guo, Chem. Nat. Compd., 55, 843 (2019).
H. Y. Yang, Y. Q. Duan, Y. K. Yang, X. Liu, L. Ye, Q. L. Mi, W. S. Kong, M. Zhou, G. Y. Yang, Q. F. Hu, X. M. Li, and J. Li, Chem. Nat. Compd., 55, 428 (2019).
S. L. Sheng, Y. P. Li, H. Y. Xiang, Y. Liu, Y. D. Wang, L. P. Kong, G. Du, Q. F. Hu, Y. J. Chen, and W. G. Wang, Rec. Nat. Prod., 14, 42 (2020).
C. O. Wilson and O. Gisvolds, Textbook of Organic Medicinal and Pharmaceutical Chemistry, Lippincott, Williams and Wilkins, Philadelphia, 2004, pp. 255–257.
S. R. M. Ibrahim and G. A. Mohamed, Phytochem. Rev., 15, 279 (2016).
C. Zhao, K. P. Rakesh, S. Mumtaz, B. Moku, A. M. Asiri, H. M. Marwani, H. M. Manukumar, and H. L. Qin, RSC Adv., 8, 9487 (2018).
L. M. Liao, Y. Q. Sun, J. Li, W. S. Kong, X. Liu, Y. Xu, H. T. Huang, W. L. Zeng, Q. L. Mi, G. Y. Yang, Q. F. Hu, and Y. K. Li, Chem. Nat. Compd., 56, 58 (2020).
Clinical and Laboratory Standards Institute, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, Approved Standard, Vol. 32, Clinical and Laboratory Standards Institute, Wayne, Pa, USA, 9th edition, 2012.
Acknowledgment
This project was supported financially by the National Natural Science Foundation of China (No. 21967021), the Foundation of Yunnan Tobacco Industry Co., Ltd. (No. 2019JC04), and the Foundation of Yunnan Innovative Research Team.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 2, March–April, 2021, pp. 252–255.
Rights and permissions
About this article
Cite this article
Li, XM., Mi, QL., Gao, Q. et al. Antibacterial Naphthalene Derivatives from the Fermentation Products of the Endophytic Fungus Phomopsis fukushii. Chem Nat Compd 57, 293–296 (2021). https://doi.org/10.1007/s10600-021-03340-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-021-03340-y