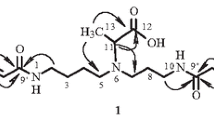
A novel 13-membered spermidine macrocyclic alkaloid, (2R)-9-furoyl-2-phenyl-1,5,9-triazacyclotridecane-4,13-dione, named celacarfurine (1), was isolated from the roots of Tripterygium wilfordii. Its structure has been elucidated on the basis of NMR and MS data. To the best of our knowledge, 13-membered spermidine macrocyclic alkaloids were rarely reported natural products; moreover, the known ones were generally in the 2S configuration and there was none or only one amide carbonyl in the macrocycle. A (2R)-13-membered spermidine macrocyclic alkaloid containing two amide carbonyls in the macrocycle (celacarfurine) is reported here for the first time. Compound 1 showed statistically significant inhibitory effects on IL-1β secretion in LPS-induced rat primary synovial fibroblasts at 10 μM.

Similar content being viewed by others
References
Fei Shen, Yongliang Bai, Shulan Su, and Jinao Duan, J. Chin. Med. Mater., 37, 1809 (2014).
Ni Lin, Ma Jie, Li Chuangjun, Li Li, Guo Jiamei, Yuan Shaopeng, and Hou Qi, China J. Chin. Mater. Med., 35, 515 (2010).
Jianqun Liu, W. U. Qiushan, and Y. U. Zhaofen, Chem. Ind. Forest Prod., 37, 72 (2017).
Jianqun Liu, Qiushan Wu, Jicheng Shu, Rui Zhang, and Lifang Liu, Fitoterapia, 120, 126 (2017).
Luo Yinggang, Pu Xiang, Luo Guoyong, Zhou Min, Ye Qi, Liu Yan, Gu Jian, Qi Huayi, Li Guoyou, and Zhang Guolin, J. Nat. Prod., 77, 1650 (2014).
S. M. Kupchan, H. P. J. Hintz, R. M. Smith, A. Karim, M. W. Cass, W. A. Court, and M. Yatagai, J. Chem. Soc. Chem. Commun., 9, 329 (1974).
Chris J. Hamilton, Ahilan Saravanamuthu, Christiane Poupat, Alan H. Fairlamb, and Ian M. Eggleston, Bioorg. Med. Chem., 14, 2266 (2006).
Gustavo da Silva, Ana Martinho, Raquel Gonzalez Soengas, Ana Paula Duarte, Rita Serrano, Elsa Teixeira Gomes, and Olga Silva, Fitoterapia, 106, 7 (2015).
P. Kuehne, A. Guggisberg, and M. Hesse, Helv. Chim. Acta, 80, 1802 (1997).
H. Duan, K. Kawazoe, M. Bando, M. Kido, and Y. Takaishi, Phytochemistry, 46, 535 (1997).
Takashi Morota, Chun-Xin Yang, Hiroshi Sasaki, Nan-Zhang Qin, Ko Sugama, Kang-Li Miao, Takaaki Yoshino, Li-Hong Xu, Masao Maruno, and Bing-Hui Yang, Phytochemistry, 39, 1153 (1995).
K. Ganesan, C. Balachandran, B. M. Manohar, and R. Puvanakrishnan, Rheumatol. Int., 32, 3181 (2012).
Acknowledgment
This research was financially supported by the National Natural Science Foundation of China (No. 81860686) and the Project for Academic and Technical Leaders of Major Disciplines in Jiangxi (No. 20182BCB22004).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 3, May–June, 2020, pp. 427–429.
Rights and permissions
About this article
Cite this article
Liu, J., Wu, Q., Shu, J. et al. A Novel Spermidine Macrocyclic Alkaloid from the Roots of Tripterygium wilfordii. Chem Nat Compd 56, 496–499 (2020). https://doi.org/10.1007/s10600-020-03070-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-020-03070-7