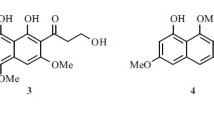
Two new naphthalene derivatives, 5-methoxy-2-methyl-7-(3-methyl-2-oxobut-3-enyl)-1-naphthaldehyde (1) and 2-(hydroxymethyl)-5-methoxy-7-(3-methyl-2-oxobut-3-enyl)-1-naphthaldehyde (2), together with two known naphthalene derivatives (3 and 4), were isolated from the fermentation products of an endophytic fungus Phomopsis sp. Their structures were elucidated by spectroscopic methods, including extensive 1D and 2D NMR techniques. Compounds 1 and 2 were evaluated for their anti-methicillin-resistant Staphylococcus aureus (anti-MRSA) activity. The results showed that compounds 1 and 2 showed good inhibition with IZD of 14.5 ± 1.2 and 15.2 ± 1.3 mm, respectively.
Similar content being viewed by others
References
M. A. Abdalla and J. C. Matasyoh, Nat. Prod. Bioprospect., 4, 257 (2014).
U. F. Castillo, G. A. Strobel, E. J. Ford, W. M. Hess, H. Porter, J. B. Jensen, H. Albert, R. Robison, M. A. Condron, D. B. Teplow, D. Stevens, and D. Yaver, Microbiology, 148, 2675 (2002).
C. Lee, S. Kim, W. Li, S. Bang, H. Lee, H. J. Lee, E. Y. Noh, J. E. Park, W. Y. Bang, and S. H. Shim, J. Antibiot. (Tokyo), (2017).
A. H. Aly, A. Debbab, and P. Proksch, Pharmazie, 68, 499 (2013).
S. Kusari, C. Hertweck, and M. Spiteller, Chem. Biol., 19, 792 (2012).
S. R. M. Ibrahim and G. A. Mohamed, Phytochemistry Rev., 15, 279 (2016).
J. R. Liou, M. Elshazly, Y. C. Du, C. N. Tseng, T. L. Hwang, Y. L. Chuang, Y. M. Hsu, P. W. Hsieh, C. C. Wu, and S. L. Chen, Phytochemistry, 88, 67 (2013).
N. Wongsa, S. Kanokmedhakul, K. Kanokmedhakul, P. Kongsaeree, S. Prabpai, and S. G. Pyne, Phytochemistry, 95, 368 (2013).
F. Liu, F. S. Li, Z. M. Feng, Y. N. Yang, J. S. Jiang, L. Li, and P. C. Zhang, Phytochemistry, 110, 150 (2015).
S. Onkar, A. Mohammed, and A. Nida, Int. J. Clin. Pharm., 2, 17 (2011).
L. Hu, N. N. Chen, Q. Hu, C. Yang, Q. S. Yang, and F. F. Wang, Molecules, 19, 11453 (2014).
H. Y. Yang, Y. H. Gao, D. Y. Niu, L. Y. Yang, X. M. Gao, G. Du, and Q. F. Hu, Fitoterapia, 91, 189 (2013).
Y. Meng, Y. Yang, Y. Qin, C. Xia, M. Zhou, X. Gao, G. Du, and Q. F. Hu, Nat. Prod. Commun., 10, 305 (2015).
N. Abdissa, F. Pan, A. Gruhonjic, J. Grafenstein, P. A. Fitzpatrick, G. Landberg, K. Rissanen, A. Yenesew, and M. Erdelyi, J. Nat. Prod., 79, 2149 (2016).
A. Kaur, H. A. Raja, G. Deep, R. Agarwal, and N. H. Oberliesa, Magn. Reson. Chem., 54, 164 (2016).
M. M. Nganga, H. Hussain, S. Chhabra, C. Langat-Thoruwa, A. Al-Harrasi, K. Krohn, and I. R. Green, Chin. Chem. Lett., 23, 576 (2012).
M. Zhou, K. Zhou, Y. L. Zhao, N. J. Xiang, T. D. Zhang, Y. D. Wang, W. Dong, B. K. Ji, L. M. Li, J. Lou, G. P. Li, and Q. F. Hu, Phytochem. Lett., 11, 245 (2015).
S. Mulla, A. Kumar, and S. Rajdev, Clinical and Laboratory Standards Institute (CLSI), Performance Standards for Antimicrobial Susceptibility Testing: Eighteenth Informational Supplement M100-S18, 3rd ed., Wayne, Pennsylvania, 2008.
Acknowledgment
This research was supported by the National Natural Science Foundation of China (Nos. 21462051, 21562049, 31360081, and 31400303), the Research Foundation of China Tobacco Company (No. 110201502006), and the Research Foundation of China Tobacco Yunnan Industrial Co., Ltd. (Nos. 2015539200340277 and 2016JC03).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 4, July–August, 2019, pp. 533–535.
Rights and permissions
About this article
Cite this article
Li, XM., Zeng, YC., Chen, JH. et al. Two New Naphthalene Derivatives from the Fermentation Products of an Endophytic Fungus Phomopsis sp.. Chem Nat Compd 55, 618–621 (2019). https://doi.org/10.1007/s10600-019-02762-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-019-02762-z