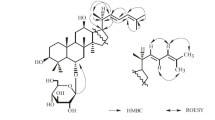
3β,12β,25-Trihydroxydammar-(E/Z)-20(22)-ene-6-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside, 3β,2β,25-trihydroxydammar-(E/Z)-20(22)-ene-6-O-β-D-glucopyranoside, and dammar-20(22)-ene-3,6,12,25-tetrol(3β,6β,12β,20E/Z) were synthesized from the ginsenosides Re, Rh1, and PPT, respectively, via a simple three-step process involving acetylation, elimination-addition, and saponification. We obtained the detailed structures of these compounds by 1D and 2D NMR, and by HR-ESI-MS. Among them, dammar-20(22)-ene-3,6,12,25-tetrol(3β,6β,12β,20Z) was identified as a new triterpenoid ginsenoside. The cytotoxic and hemolytic effects of these compounds towards cancer cells and erythrocytes were also evaluated.


Similar content being viewed by others
References
I. H. Cho, H. J. Lee, and Y. S. Kim, J. Agric. Food. Chem., 60, 7616 (2012).
T. Tani, M. Kubo, T. Katsuki, M. Hinashino, and H. Teruaki, J. Nat. Prod., 44, 401 (1981).
O. Sticher, Phytomed. Eur., 16, 221 (1998).
J. M. Lu, Q. Yao, and C. Chen, Curr. Vasc. Pharmacol., 17, 293 (2009).
K. L. Ge, W. F. Chen, J. X. Xie, and M. S. Wong, J. Ethnopharmacol., 127, 118 (2010).
Y. Wei, C. M. Ma, and M. Hattori, Phytochem. Lett., 2, 63 (2009).
Y. J. Kim, N. Yamabe, P. Choi, J. W. Lee, J. Ham, and K. S. Kang, J. Agric. Food. Chem., 61, 9185 (2013).
L. N. Tao, Q. Meng, J. Y. Yin, R. Xing, and H. R. Guo, Chin. Chem. Lett., 20, 687 (2009).
G. Chen, Y. Song, L. Wu, and D. Guo, J. Nat. Prod., 70, 1203 (2007).
L. Y. Ma and X. W. Yang, J. Phytochem. Lett., 20, 13 (2015).
G. T. Qian and Y. P. Chen, Steroids, 92, 1 (2014).
T. Mosmann, J. Immunol. Methods, 65, 55 (1983).
Acknowledgment
The authors are grateful to the State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, University of Jilin at Changchun for access to the Bruker Avance-500 NMR instrument used in this study. Other chemical analysis was provided by the Alan. G. MacDiarmid Laboratory of Jilin University. We thank James Monypenny, PhD, from Edanz Group, for editing the English text of a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 1, January–February, 2019, pp. 59–64.
Rights and permissions
About this article
Cite this article
Ding, H., Chen, Y., Chen, S. et al. Semi-Synthesis and Cellular Effects of Three Different Ginsenosides Derived from Re, Rh1, and PPT. Chem Nat Compd 55, 66–73 (2019). https://doi.org/10.1007/s10600-019-02615-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-019-02615-9