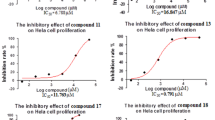
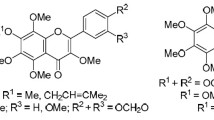
Two series of polymethoxychalcones and polymethoxyflavones, including the natural products 2′-hydroxy-3,4,5,4′,6′-pentamethoxychalcone (8c), 5,6,7,8,3′,4′,5′-heptamethoxyflavone (6), 5,7,3′,4′,5′-pentamethoxyflavone (9c), 3-hydroxy-5,6,7,8,3′,4′,5′-heptamethoxyflavone (7), and 3-hydroxy-5,7,3′,4′,5′-pentamethoxyflavone (10), were synthesized. The antiproliferative activity in vitro was evaluated against a panel of three human cancer cell lines (HeLa, HCC1954, and SK-OV-3) by the cell counting kit-8 (CCK-8) assay. The results showed that most of the synthetic compounds exhibited moderate to potent antiproliferative activities. Some compounds displayed equal or higher potential than the positive control drug cisplatin. In particular, compounds 4c, 4e, 8a, and 9a possess IC50 values equal to or below 10 μM and are worthy of further investigation.
Similar content being viewed by others
References
M. Singh, M. Kaur, and O. Silakari, Eur. J. Med. Chem., 84, 206 (2014).
Y. Yanqing, D. Wei, L. Chunbo, L. Ping, S. Qinpeng, Y. Juanxia, W. Yuede, Z. Kun, J. Bingkun, G. Xuemei, Z. Min, and H. Qiufen, Chem. Nat. Compd., 52, 359 (2016).
T. K.-D. Hoang, T. K.-C. Huynh, and T. -D. Nguyen, Bioorg. Chem., 63, 45 (2015).
D. Mahapatra, S. K. Bharti, and V. Asati, Eur. J. Med. Chem., 98, 69 (2015).
N. Bathelemy, W. F. Ghislain, A. Pantaleon, K. Justin, D. Arif, and T. N. Bonaventure, Chem. Nat. Compd., 53, 207 (2017).
Y. Miyata, T. Sshitari, Y. Okuyama, A. Schimada, H. Takahashi, H. Natsugari, and H. Kosano, Bioorg. Med. Chem. Lett., 23, 183 (2013).
B. P. Bandgar, S. S. Gawande, R. G. Bodade, and J. V. Totre, Bioorg. Med. Chem., 18, 1364 (2010).
T. Walle, N. Ta, T. Kawamori, X. Wen, P. A. Tsuji, and V. Walle, Pharmacology, 73, 1288 (2007).
S. Kawaii, T. Ikuina, T. Hikima, T. Tokiwano, and Y. Yoshizawa, Anticancer Res., 32, 5239 (2012).
S. Li, M.-H. Pan, C.-S. Lai, C.-Y. Lo, S. Dushenkov, and C.-T. Ho, Bioorg. Med. Chem., 15, 3381 (2007).
V.-S. Nguyen, L. Shi, F.-Q. Luan, and Q.-A. Wang, Acta Biochim. Pol., 62, 547 (2015).
V.-S. Nguyen, W. Li, Y. Li, and Q.-A. Wang, Med. Chem. Res., 26, 1585 (2017).
V.-S. Nguyen, L. Shi, S.-C. Wang, and Q.-A. Wang, Anti-cancer Agents Med. Chem., 17, 134 (2016).
A. Miroslaw, S. Katarzyna, and Z. Anna, Tetrahedron, 64, 9544 (2008).
P. N. Marta, T. L. Raquel, C. Kanthima, P. Panee, S. J. N. Maria, V. M. Helena, P. Madalena, M. S. S. Artur, and C. Honorina, Chem. Biodivers., 9, 1133 (2012).
L.-V. Ngo and T. V. C. Pham, Phytochemistry, 18, 1859 (1979).
M. J. Mashimbye, P. Soundy, and R. T. Van, J. Chem., 59, 1 (2006).
K. Takeshi and F. Kurnia, Phytochemistry, 45, 179 (1997).
S. L. Cai, S. Liu, L. Liu, and Q. A. Wang, Chem. Res. Chin. Univ., 28, 631 (2012).
A. Detsi, M. Majdalani, C. A. Kontogiorgis, H. L. Dimitra, and P. Kefalas, Bioorg. Med. Chem., 17, 8073 (2009).
Q. A. Wang, Z. Wu, L. Liu, L. H. Zou, and M. Luo, Chin. J. Org. Chem., 30, 1682 (2010).
M. Tsukayama, E. Kusunoki, and M. M. Hossain, Heterocycles, 71, 1589 (2007).
Y. P. Song, Z. Y. Xin, Y. M. Wan, J. B. Li, B. P. Ye, and X. W. Xue, Eur. J. Med. Chem., 90, 695 (2015).
Acknowledgment
We thank the National Natural Science Foundation of China (No. J1210040, 21173074) for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 1, January–February, 2019, pp. 12–17.
Rights and permissions
About this article
Cite this article
Vongdeth, K., Han, P., Li, W. et al. Synthesis and Antiproliferative Activity of Natural and Non-Natural Polymethoxychalcones and Polymethoxyflavones. Chem Nat Compd 55, 11–17 (2019). https://doi.org/10.1007/s10600-019-02605-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-019-02605-x