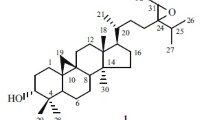
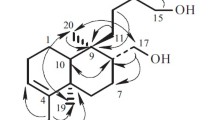
A new pregnane-type steroid, 2α,3β,16β-trihydroxy-5α-pregnane-20R-methacrylate (1), was isolated from the leaves of Melia azedarach, together with 17 known compounds. Their structures were elucidated by spectroscopic and mass-spectrometric analysis, including 1D, 2D NMR, ESI-MS, and HR-MS. Compound 1 was found to possess cytotoxicities against HepG2, K562, and SGC7901 cell lines, with IC50 values 18.6, 19.1, and 62.9 μM, respectively.

Similar content being viewed by others
References
H. B. Liu, C. R. Zhang, S. H. Dong, L. Dong, Y. Wu, and J. M. Yue, J. Am. Chem. Soc., 59, 1003 (2011).
T. Namba, The Encyclopedia of Wakan-Yaku (Traditional Sino-Japanese Medicines) with Color Pictures, Hoikusya, Osaka, 1994, 247 pp.
M. Okada, Newly Revised Illustrated Medicinal Plants of World, Hokuryukan Publishing Co., Ltd., Tokyo, 2002, 262 pp.
M. D. Ambrosio and A. Guerriero, Phytochemistry, 60, 419 (2002).
W. M. Zhang, J. Q. Liu, X. R. Peng, L. S. Wan, and Z. R. Zhang, Nat. Prod. Biopros., 4, 157 (2014).
Z. X. Zhang, J. Zhou, K. Hayashi, and K. Kaneko, Phytochemistry, 27, 2935 (1988).
L. Garrido, E. Zubia, M. J. Ortega, and J. Salva, Steroids, 65, 85 (2000).
X. D. Luo, S. H. Wu, Y. B. Ma, and D. G. Wu, Chin. Chem. Lett., 11, 535 (2000).
R. Benvegnu, G. Cimino, S. D. Rosa, and S. D. Stefano, Experientia, 38, 1443 (1982).
W. R. Nes, T. E. Varkey, D. R. Crump, and M. Gut, J. Org. Chem., 41, 3429 (1976).
W. R. Nes and T. E. Varkey, J. Org. Chem., 41, 1652 (1976).
A. Aiello, E. Fattorusso, and M. Menna, Steroids, 56, 513 (1991).
M. Nakatani, H. Takao, I. Miura, and T. Hase, Phytochemistry, 24, 1945 (1985).
S. B. Wu, Y. P. Ji, J. J. Zhu, Y. Zhao, G. Xia, Y. H. Hu, and J. F. Hu, Steroids, 74, 761 (2009).
P. He, S. Li, S. J. Wang, Y. C. Yang, and J. G. Shi, Chin. J. Chin. Mater. Med., 30, 671 (2005).
X. L. Liu, M. S. Dong, X. H. Chen, M. Jiang, X. Lv, and G. Yan, Food Chem., 105, 548 (2007).
F. Conforti, C. Statti, M. R. Loizzo, G. Sacchetti, F. Poli, and F. Menichini, Bio. Pharm. Bull., 28, 1098 (2005).
L. Tang, Y. C. Chen, Z. B. Jiang, S. P. Zhong, W. Z. Chen, W. D. Chen, Y. J. Zhuang, and G. G. Shi, Chem. Nat. Compd., 53, 770 (2017).
C. Gobel and I. Feussner, Phytochemistry, 70, 1485 (2009).
M. Saleem, Cancer Lett., 285, 109 (2009).
Y. J. You, N. H. Nam, Y. Kim, K. H. Bae, and B. Z. Ahn, Phytother. Res., 17, 341 (2003).
X. H. Gan, X. Zhou, H. G. Chen, X. J. Gong, J. X. Zhang, and D. P. Wang, J. Trop. Subtrop. Bot., 17, 160 (2009).
S. Matsunaga, R. Tanaka, and M. Akagi, Phytochemistry, 27, 535 (1988).
M. F. Otuki, J. Ferrerira, F. V. Lima, C. M. Silva, A. Malhriros, L. A. Muller, C. S. Cani, A. R. S. Santos, R. A. Yunes, and J. B. Calixto, J. Pharmacol. Exp. Ther., 313, 310 (2005).
J. Y. Kim, H. J. Lim, and J. H. Ryu, Bioorg. Med. Chem. Lett., 18, 1511 (2008).
J. Budzianowski and L. Skrzypczak, Phytochemistry, 38, 997 (1995).
L. J. Mcgaw, A. K. Jager, and J. Staden, Fitoterapia, 73, 431 (2002).
B. J. B. Wood, B. W. Nichols, and A. T. James, BBA-Lipids and Lipids Metabolism, 106, 261 (1965).
A. C. Chibnall, E. F. Willians, A. L. Latner, and S. H. Piper, Biochem. J., 27, 1885 (1933).
A. Pollard, A. C. Chibnall, and S. H. Piper, Biochem. J., 27, 1889 (1933).
M. R. Habib, F. Nikkon, M. Rahman, M. E. Haque, and M. R. Karim, J. Biol. Sci., 10, 4174 (2007).
Z. J. Ma, X. Li, N. Li, and J. H. Wang, Fitoterapia, 73, 313 (2002).
K. Tabata, K. Motani, N. Takayanagi, R. Nishimura, S. Asami, Y. Kimura, M. Ukiya, D. Hasegawa, T. Akihisa, and T. Suzuki, Biol. Pharm. Bull., 28, 1404 (2005).
T. Kikuchi, E. Uchiyama, M. Ukiya, K. Tabata, Y. Kimura, T. Suzuki, and T. Akihisa, J. Nat. Prod., 74, 137 (2011).
Acknowledgment
This work was supported by grants from the Natural Science Foundation of Fujian Province (No. 2016J01369), the Open Project of National Marine Bureau Key Laboratory of Marine Biogenetic Resources (HY201506, HY201604), and the Startup Fund for scientific research, Fujian Medical University (2016QH015). The authors are grateful to State Key Laboratory of Applied Organic Chemistry at Lanzhou University for measuring of NMR and mass spectra.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 5, September–October, 2018, pp. 781–784.
Rights and permissions
About this article
Cite this article
Ma, X., Zhou, F., Deng, Y. et al. A New Steroid Ester from the Leaves of Melia azedarach. Chem Nat Compd 54, 921–925 (2018). https://doi.org/10.1007/s10600-018-2513-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-018-2513-x