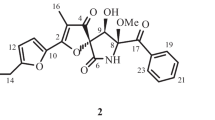
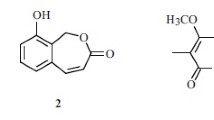
Methyl (3-hydroxy-4-(2-hydroxy-6-methylheptan-2-yl)benzoyl)glycinate (1), a new sydonic acid derivative with glycinate, together with sydonic acid (2), sydowic acid (3), and 7-deoxy-7,14-didehydrosydonic acid (4), were isolated from a marine-derived Aspergillus sydowii strain CUGB-F126. Their structures were elucidated by spectroscopic analysis, including high-resolution mass spectroscopy, and 1D and 2D NMR techniques. All of these compounds did not show inhibitory activity against Staphylococcus aureus and Candida albicans.
Similar content being viewed by others
References
T. F. Patterson, W. R. Kirkpatrick, M. White, J. W. Hiemenz, J. R. Wingard, B. Dupont, M. G. Rinaldi, D. A. Stevens, and J. R. Graybill, Medicine (Baltimore), 79, 250 (2000).
A. Yamada, H. Noguchi, H. Sakae, T. Sugita, M. Hiruma, and M. Hiruma, Med. Mycol. J., 53, 205 (2012).
G. W. Smith, L. D. Ives, I. A. Nagelkerken, and K. B. Richie, Nature, 383, 487 (1996).
K. Kim and C. D. Harvell, Am. Nat., 164, S52 (2004).
T. Hamasaki, Y. Sato, and Y. Hatsuda, Agric. Biol. Chem., 39, 2337 (1975).
T. Hamasaki, K. Nagayama, and Y. Hatsuda, Agric. Biol. Chem., 42, 40 (1978).
K. Trisuwan, V. Rukachaisirikul, M. Kaewpet, S. Phongpaichit, N. Hutadilok-Towatana, S. Preedanon, and J. Sakayaroj, J. Nat. Prod., 74, 1663 (2011).
X. Liu, F. Song, L. Ma, C. Chen, X. Xiao, B. Ren, X. Liu, H. Dai, A. M. Piggott, Y. Av-Gay, L. Zhang, and R. J. Capon, Tetrahedron Lett., 54, 6081 (2013).
A. Kaur, H. A. Raja, B. A. Darveaux, W. L. Chen, S. M. Swanson, C. J. Pearce, and N. H. Oberlies, Magn. Reson. Chem., 53, 616 (2015).
M. Y. Wei, C. Y. Wang, Q. A. Liu, C. L. Shao, Z. G. She, and Y. C. Lin, Mar. Drugs, 8, 941 (2010).
Acknowledgment
This work was supported by the Fundamental Research Funds for the Central Universities (2-9-2015-165) and key lab of marine bioactive substance and modern analytical technique, SOA (MBSMAT-2016-04).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 6, November–December, 2017, pp. 898–899.
Rights and permissions
About this article
Cite this article
Xu, X., Zhao, S., Yin, L. et al. A New Sydonic Acid Derivative From a Marine Derived-Fungus Aspergillus sydowii . Chem Nat Compd 53, 1056–1058 (2017). https://doi.org/10.1007/s10600-017-2200-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-017-2200-3