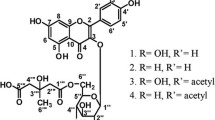
Flavones are a group of plant secondary metabolites with multiple biological properties. In the present study, 5,6,7,8-tetrahydroxyflavone (5,6,7,8-THF) was synthesized and characterized. In vitro antioxidant study, the effect of 5,6,7,8-THF on total antioxidant activity, reducing power, DPPH radical scavenging, ABTS radical scavenging, superoxide radical scavenging, hydroxyl radical scavenging, nitric oxide radical scavenging, and ferrous chelating activities was examined. According to the results, 5,6,7,8-THF showed excellent free radical scavenging effect and reducing power but weak the ferrous chelating activity. In conclusion, 5,6,7,8-THF can be regarded as an excellent source of antioxidants.


Similar content being viewed by others
References
M. Singh, M. Kaur, and O. Silakari, Eur. J. Med. Chem., 84, 206 (2014).
J. Greeff, J. Joubert, S. F. Malan, and S. van Dyk, Bioorg. Med. Chem., 20, 809 (2012).
O. Firuzi, A. Lacanna, R. Petrucci, G. Marrosu, and L. Saso, Biochim. Biophys. Acta, 1721, 174 (2005).
Y. H. Chen, Z. S. Yang, C. C. Wen, Y. S. Chang, B. C. Wang, C. A. Hsiao, and T. L. Shih, Food Chem, 134, 717 (2012).
S. A. van Acker, D. J. van den Berg, M. N. Tromp, D. H. Griffioen, W. P. van Bennekom, W. J. van der Vijgh, and A. Bast, Free Radic. Biol. Med., 20, 331 (1996).
N. Krafczyk, F. Woyand, and M. A. Glomb, Mol. Nutr. Food Res., 53, 635 (2009).
A. I. Khlebnikov, I. A. Schepetkin, and M. T. Quinn, Bioorg. Med. Chem., 16, 2791 (2008).
A. T. Girgih, R. He, F. M. Hasan, C. C. Udenigwe, T. A. Gill, and R. E. Aluko, Food Chem., 173, 652 (2015).
J. Taira, E. Tsuchida, M. C. Katoh, M. Uehara, and T. Ogi, Food Chem., 166, 531 (2015).
A. M. Abbasi, M. H. Shah, T. Li, X. Fu, X. Guo, and R. H. Liu, J. Ethnopharmacol., 162, 333 (2015).
P. Prieto, M. Pineda, and M. Aguilar, Anal. Biochem., 269, 337 (1999).
H. Park, T. T. Dao, and H. P. Kim, Eur. J. Med. Chem., 40, 943 (2005).
P. Bovicelli, V. D′Angelo, D. Collalto, A. Verzina, N. D′Antona, and D. Lambusta, J. Pharm. Pharmacol., 59, 1697 (2007).
J. F. W. McOmie, M. L. Watts, and D. E. West, Tetrahedron, 24, 2289 (1968).
J. A. Vaz, L. Barros, A. Martins, C. Santos-Buelga, M. H. Vasconcelos, and I. C. F. R. Ferreira, Food Chem., 126, 610 (2011).
R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, and C. Rice-Evans, Free Radic. Biol. Med., 26, 1231 (1999).
J. Liu, L. Jia, J. Kan, and C. H. Jin, Food Chem. Toxicol., 51, 310 (2013).
R. A. Khan, M. R. Khan, S. Sahreen, and M. Ahmed, Chem. Cent. J., 6, 12 (2012).
B. Sangameswaran, B. R. Balakrishnan, C. Deshraj, and B. Jayakar, Pak. J. Pharm. Sci., 22, 368 (2009).
J. He, B. Huang, X. Ban, J. Tian, L. Zhu, and Y. Wang, J. Ethnopharmacol., 141, 104 (2012).
M. Oyaizu, Jpn. J. Nutr., 44, 307 (1986).
Acknowledgment
Financial support from the National Natural Science Foundation of China (81202458), China Postdoctoral Science Foundation (2012M521926), and Scientific Research Foundation of Gansu Province (1308RJYA061) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 2, March–April, 2017, pp. 213–217.
Rights and permissions
About this article
Cite this article
Jing, L., Ma, H., Fan, P. et al. Synthesis and Antioxidant Properties of 5,6,7,8-Tetrahydroxyflavone. Chem Nat Compd 53, 248–253 (2017). https://doi.org/10.1007/s10600-017-1963-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-017-1963-x