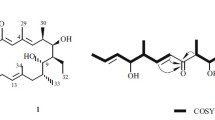
A new anthraquinone, 7-(γ,γ)-dimethylallyloxymacrosporin (1), along with five known analogues, macrosporin (2), 7-methoxymacrosporin (3), tetrahydroaltersolanol B (4), altersolanol L (5), and ampelanol (6), were isolated from the mangrove endophytic fungus Phoma sp. L28. All of them were first found in Phoma sp. Their structures were established by comprehensive spectroscopic analyses and comparison with the published data. These compounds displayed in vitro antifungal activities against Colletotrichum musae (Berk. & M. A. Curtis) Arx., Colletotrichum gloeosporioides (Penz) Sacc., Fusarium graminearum Schw., Penicillium italicum Wehme, Fusarium oxysporum Schlecht. f. sp. lycopersici (Sacc.) W.C. Snyder et H. N. Hansen, and Rhizoctonia solani Kuhn at different levels. Notably, compound 2 exhibited potent antifungal activity against Fusarium graminearum Schw. as compared with the positive control carbendazim.
Similar content being viewed by others
References
E. Montel, P. D. Bridge, and B. C. Sutton, Mycopathologia, 115, 89 (1991).
E. L. Kim, J. L. Li, H. T. Dang, J. Hong, C. O. Lee, D. K. Kim, W. D. Yoon, E. Kim, Y. H. Liu, and J. H. Jung, Bioorg. Med. Chem. Lett., 22, 3126 (2012).
Y. Kanai, D. Ishiyama, H. Senda, W. Iwatani, H. Takahashi, H. Konno, S. Tokumasu, and S. Kanazawa, J. Antibiot., 53, 863 (2000).
K. Herath, G. Harris, H. Jayasuriya, D. Zink, S. Smith, F. Vicente, G. Bills, J. Collado, A. Gonzalez, B. Jiang, J. N. Kahn, S. Galuska, R. Giacobbe, G. Abruzzo, E. Hickey, P. Liberateor, D. Xu, T. Roemer, and S. B. Singh, Bioorg. Med. Chem., 17, 1361 (2009).
S. Huang, W. J. Ding, C. Y. Li, and D. G. Cox, Pharmacogn. Mag., 10, 410 (2014).
W. J. Ding, S. Q. Zhang, B. Gong, C. Y. Li, and X. F. Wang, Guangdong Agric. Sci., 41, 74 (2014).
A. H. Aly, R. A. E. Ebel, V. Wary, W. E. G. Muller, S. Kozytska, U. Hentschel, P. Proksch, and R. Ebel, Phytochemistry, 69, 1716 (2008).
A. Evidente, R. Rodeva, A. Andolfi, Z. Stoyanova, C. Perrone, and A. Motta, Eur. J. Plant Pathol., 130, 173 (2011).
N. Okamura, K. Mimura, H. Haraguchi, K. Shingu, K. Miyahara, and A. Yagi, Phytochemistry, 42, 77 (1996).
A. Debbab, A. H. Aly, R. A. E. Ebel, V. Wray, W. E. G. Muller, F. Totzke, U. Zirrgiebel, C. Schachtele, M. H. G. Kubbutat, W. H. Lin, M. Mosaddak, A. Hakiki, P. Proksch, and R. Ebel, J. Nat. Prod., 72, 626 (2009).
X. M. Zhou, C. J. Zheng, G. Y. Chen, X. P. Song, C. R. Han, G. N. Li, Y. H. Fu, W. H. Chen, and Z. G. Niu, J. Nat. Prod., 77, 2021 (2014).
Y. N. Wang, C. J. Zheng, C. L. Shao, and C. Y. Wang, Chin. J. Mar. Drugs, 34, 10 (2015).
C. J. Zheng, C. L. Shao, Z. Y. Guo, J. F. Chen, D. S. Deng, K. L. Yang, Y. Y. Chen, X. M. Fu, Z. G. She, Y. C. Lin, and C. Y. Wang, J. Nat. Prod., 75, 189 (2012).
J. H. Wang, S. Huang, C. Y. Li, W. J. Ding, Z. G. She, and C. L. Li, Chem. Nat. Compd., 51, 239 (2015).
S. Prachya, S. Wiyakrutta, N. Sriubolmas, N. Ngamrojanavanich, C. Mahidol, S. Ruchirawat, and P. Kittakoop, Planta Med., 73, 1418 (2007).
J. H. Wang, W. J. Ding, R. M. Wang, Y. P. Du, H. L. Liu, X. H. Kong, and C. Y. Li, Mar. Drugs, 13, 4492 (2015).
Acknowledgment
This work was supported by the National Natural Science Foundation of China (21102049), the Natural Science Foundation of Guangdong Province of China (2015A030313405, 9451064201003751), the Science and Technology Project of Guangdong Province (2016A020222019), the Scientific Research Foundation for Returning Overseas Chinese Scholars, State Education Ministry 2015-311, and the Innovation Experiment Program for University students of Guangdong Province (201510564196).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 2, March–April, 2017, pp. 204–206.
Rights and permissions
About this article
Cite this article
Huang, S., Xu, J., Li, F. et al. Identification and Antifungal Activity of Metabolites from the Mangrove Fungus Phoma sp. L28. Chem Nat Compd 53, 237–240 (2017). https://doi.org/10.1007/s10600-017-1961-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-017-1961-z