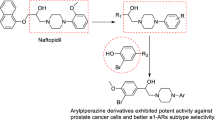
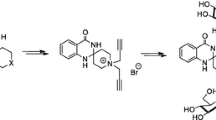
Synthesized ligustrazine derivatives (1a, 1b, 1c (novel)) were structurally confirmed by mass spectrometry, 1H NMR, and 13C NMR. The cytotoxic activities of all derivatives were evaluated by MTS assay in three human prostate cancer cell lines (PC-3, LNCaP, and DU145) and in the A549 human lung cancer cell line. Compound 1a exhibited strong cytotoxic activity against PC-3 cells (IC50 3.63 μM). In addition, compounds 1–1c showed moderate α 1-adrenergic receptor (AR) subselective antagonistic and β 2-AR agonistic effects, indicating potential use for the treatment of chronic obstructive pulmonary disease. Molecular docking results showed 1b bound to one region of the active site in β 2-AR, but 1, 1a, and 1c bound to a different region.
Similar content being viewed by others
References
X. C. Cheng, X. Y. Liu, W. F. Xu, X. L. Guo, and Y. Ou, Bioorg. Med. Chem., 15, 3315 (2007).
C. C. Tsai, T. Y. Lai, W. C. Huang, I. M. Liu, and J. T. Cheng, Life Sci., 71, 1321 (2002).
X. L. Zhu, L. Z. Xiong, Q. Wang, Z. G. Liu, X. Ma, Z. H. Zhu, S. Hu, G. Gong, and S. Y. Chen, Neurosci Lett., 449, 24 (2009).
D. H. Wu, X. J. Xu, M. Z. Zhang, and L. Wang, Lett. Drug Des. Discov., 8, 1009 (2011).
R. Barbaro, L. Betti, M. Botta, F. Corelli, G. Giannaccini, L. Maccari, F. Manetti, G. Strappaghetti, and F. Corsano, J. Med. Chem., 44, 2118 (2001).
S. C. Sato, T. Hatanaka, H.Yuyama, M. Ukai, Y. Noguchi, A. Ohtake, K. Taguchi, M. Sasamata, and K. Miyata, Biol. Pharm. Bull., 35, 72 (2012).
Sybyl Molecular Modeling Software Packages, V 8.1.1, TRIPOS Inc., St Louis, 2009.
V. Cherezov, D. M. Rosenbaum, M. A. Hanson, S. G. Rasmussen, F. S. Thian, T. S. Kobilka, H. J. Choi, P. Kuhn, W. I. Weis, B. K. Kobilka, and R. C. Stevens, Science, 318, 1258 (2007).
Acknowledgment
This work was supported by the Department of Education of Guangdong Province scientific research projects fund (Nos. 2013KJCX0151 and 2014KTSCX096).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 1, January–February, 2017, pp. 96–98.
Rights and permissions
About this article
Cite this article
Zhang, C., Chen, Ld., Liang, Xt. et al. Synthesis and Biological Evaluation of Ligustrazine Derivatives. Chem Nat Compd 53, 114–117 (2017). https://doi.org/10.1007/s10600-017-1922-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-017-1922-6