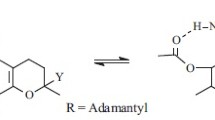
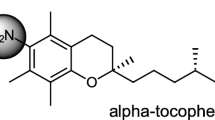
Spin-labeled α-tocopherol succinyl derivatives were synthesized by the reaction of α-tocopherol succinate with 4-amino-2,2,6,6-tetramethylpiperidine-1-oxyl, 3-aminomethyl-2,2,5,5-tetramethylpyrrolidine-1-oxyl, and 3-amino-2,2,5,5-tetramethylpyrrolidine-1-oxyl. Acylation of spin-labeled trolox amides by succinic anhydride produced the corresponding spin-labeled trolox amide succinyl derivatives.


Similar content being viewed by others
References
J. Gruber, K. Staniek, C. Krewenka, R. Moldzio, A. Patel, S. Bohmdorfer, T. Rosenau, and L. Gille, Bioorg. Med. Chem., 22, 684 (2014); V. Panda, P. Khambat, and S. Patil, Int. J. Clin. Med., 2, 515 (2011); L. Prochazka, S. Koudelka, L. Dong, J. Stursa, J. Goodwin, J. Neca, J. Slavik, M. Ciganek, J. Masek, K. Kluckova, M. Nguyen, J. Turanek, and J. Neuzil, Apoptosis, 18, 286 (2013); S. Hama, S. Utsumi, Y. Fukuda, K. Nakayama, Y. Okamura, H. Tsuchiya, K. Fukuzawa, H. Harashima, and K. Kogure, J. Controlled Release, 161, 843 (2012); A. A. Fattah, H. A. Darwish, N. Fathy, A. Raafat, and S. A. Shouman, Med. Chem. Res., 21, 2735 (2012).
K. N. Prasad, B. Kumar, X. Yan, A. J. Hanson, and W. C. Cole, J. Am. Coll. Nutr., 22 (2), 108 (2003).
J. Neuzil, X. Wang, Y. Zhao, and K. Wu, in: Nutrition and Cancer Prevention, CRC Press, Taylor & Francis, 2006, pp. 111–137.
J.-H. Lee, H.-N. Kim, D. Yang, K. Jung, H.-M. Kim, H.-H. Kim, H. Ha, and Z.-H. Lee, J. Biol. Chem., 284 (20), 13725 (2009).
S. Stvolinsky, K. Toropova, M. Gordeeva, V. Kazey, T. Sato, K. Meguro, and A. Boldyrev, Amino Acids, 43, 165 (2012); S. M. Ahn, H. S. Rho, H. S. Baek, Y. H. Joo, Y. D. Hong, S. S. Shin, Y. Park, and S. N. Park, Bioorg. Med. Chem. Lett., 21, 7466 (2011).
K. Shimizu, R. Kondo, K. Sakai, N. Takeda, T. Nagahata, and T. Oniki, Lipids, 36 (12), 1321 (2001).
Yu. V. Yushkova, E. I. Chernyak, Yu. F. Polienko, Yu. V. Gatilov, S. V. Morozov, and I. A. Grigor′ev, Chem. Nat. Compd., 49, 253 (2013).
M. Birringer, J. H. EyTina, B. A. Salvatore, and J. Neuzil, Br. J. Cancer, 88, 1948 (2003).
W. Wichitnithad, U. Nimmannit, S. Wacharasindhu, and P. Rojsitthisak, Molecules, 16, 1888 (2011).
C. W. Lee, N. H. Park, J. W. Kim, B. H. Um, A. V. Shpatov, E. E. Shults, I. V. Sorokina, and S. A. Popov, Russ. J. Bioorg. Chem., 38 (3), 328 (2012).
B. D. Anderson and V. J. Taphouse, Pharm. Sci., 70, 181 (1981); E. R. Garrett, J. Pharm. Sci., 51, 445 (1962); D. A. Brent, P. Chandrasurin, A. Ragouzeos, B. S. Hurlbert, and J. T. Burke, J. Pharm. Sci., 69, 906 (1980); M. Johansen and C. Larsen, Int. J. Pharm., 21, 201 (1984); M. A. La-Scalea, C. M. S. Menezes, G. C. Masutami, M. C. Polli, S. H. P. Serrano, and E. I. Ferreira, Electrochim. Acta, 51, 5103 (2006).
F. Mazzini, M. Betti, B. Canonico, T. Netscher, F. Luchetti, S. Papa, and F. Galli, ChemMedChem., 5, 540 (2010).
V. Anbharasi, N. Cao, and S. Feng, J. Biomed. Mater. Res., 94, 730 (2010).
Yu. F. Polienko, V. I. Vinogradova, Sh. Sh. Sagdullaev, N. D. Abdullaev, Yu. V. Gatilov, and I. A. Grigor′ev, Chem. Nat. Compd., 49, 311 (2013).
A. N. Antimonova, N. I. Petrenko, E. E. Shults, Yu. F. Polienko, M. M. Shakirov, I. G. Irtegova, M. A. Pokrovsky, K. M. Sherman, I. A. Grigor′ev, A. G. Pokrovsky, and G. A. Tolstikov, Russ. J. Bioorg. Chem., 39 (2), 181 (2013).
Acknowledgment
The work was supported financially by a grant of the RFBR No. 12-03-00718a and a Moscow Administration Program (Contract No. 16/12-Gen-M). Spectral characteristics of the synthesized compounds were obtained at the Chemical Service Center of the Novosibirsk Institute of Organic Chemistry, SB, RAS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 5, September–October, 2014, pp. 718–722.
Rights and permissions
About this article
Cite this article
Yushkova, Y.V., Chernyak, E.I., Morozov, S.V. et al. First Spin-Labeled α-Tocopherol and Trolox Succinyl Derivatives . Chem Nat Compd 50, 827–831 (2014). https://doi.org/10.1007/s10600-014-1093-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-014-1093-7