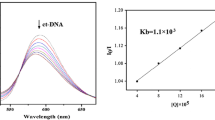
Eleven new 2-O-side-chain derivatives of alizarin were synthesized via esterification, substitution, hydrolysis, or elimination reactions. The structures of all the products were confirmed by 1H NMR, MS, and elemental analysis. Compared with alizarin, most of the derivatives had significantly higher DNA binding affinity based on interaction with ct-DNA. In particular, compound 8 exhibited the best cytotoxicity against HeLa cells with IC50 20 μM and could induce HeLa cells apoptosis.

Similar content being viewed by others
References
D. Hemen and L. Lalita, Indian J. Nat. Prod. Res., 9, 291 (2012).
R. S. Geetanjali and S. M. S. Chauhan, Chem. Biodivers., 1, 1241 (2004).
P. Kaur, M. Chandel, S. Kumar, N. Kumar, B. Singh, and S. Kaur, Food Chem. Toxicol., 48, 320 (2010).
F. Kalyoncu, B. Cetin, and H. Saglam, Phytother. Res., 20, 490 (2006).
G. C. Yen, P. D. Duh, and D. Y. Chuang, Food Chem., 70, 437 (2000).
S. V. Sharma, S. Sankar, and B. Suresh, Indian J. Exp. Biol., 40, 739 (2002).
M. T Makhija, Curr. Med. Chem., 13, 2429 (2006).
R. F. Jin, H. Z. Bao, Y. Bai, and X. H. Li, Interdisciplinary Research and Applications in Bioinformatics, Computational Biology, and Environmental Sciences, IGI Global, 2011, p. 130.
H. S. Bodiwala, A. Karmase, P. J. Deshpande, A. Kaur, N. Ahmed, S. K. Chauthe, K. G. Brahmbhatt, R. U. Phadke, D. Mitra, K. K. Bhutani, and I. P. Singh, J. Nut. Med., 65, 662 (2011).
J. Deng, T. Sanchez, N. Neamati, and J. M. Briggs, J. Med. Chem., 49, 1684 (2006).
T. V. Kharlamova, Chem. Nat. Compd., 45, 629 (2009).
J. H. Lee, N. W. Kim, E. Her, B. K. Kim, W. S. Choi, K. H. Huang, D. K. Choi, B. O. Lim, J. W. Han, and Y. M. Kim, J. Pharm. Pharmacol., 58, 503 (2006).
C. Fotia, S. Avnet, D. Granchi, and N. Baldini, J. Orthop. Res., 30, 1486 (2012).
T. Konoshima and M. Kozuka, J. Nat. Prod., 52, 987 (1989).
J. H. Tan, Q. X. Zhang, Z. S. Huang, Y. Chen, X. D. Wang, L. Q. Gu, and J. Y. Wu, Eur. J. Med. Chem., 41, 1041 (2006).
T. V. Kharlamova, Chem. Nat. Compd., 43, 391 (2007).
C. Qiao, S. Bi, Y. Sun, D. Song, H. Zhang, and W. Zhou, Spectrochim. Acta, Part A, 70, 136 (2008).
D. T. Breslin, J. E. Coury, J. R. Anderson, L. McFail-Isom, Y. Kan, L. D. Williams, L. A. Bottomley, and G. B. Schuster, J. Am. Chem. Soc., 119, 5043 (1997).
Acknowledgment
This study was supported by the 973 project (No. 2011CB512005, 2012CB723501), the National Natural Science Foundation of China (No. 81260472, 21101035), the Guangxi Natural Science Foundation of China (2011GXNSFD018010 and No. 2010GXNSFF013001), the Bagui Scholar project, and the Foundation of Ministry of Education Innovation Team (No. IRT1225).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 2, March–April, 2014, pp. 215–218.
Rights and permissions
About this article
Cite this article
Yao, G., Ye, M., Dai, W. et al. Synthesis, Cytotoxicity, DNA Binding, and Apoptosis of Alizarin 2-O-Side-Chain Derivatives. Chem Nat Compd 50, 242–246 (2014). https://doi.org/10.1007/s10600-014-0922-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-014-0922-z