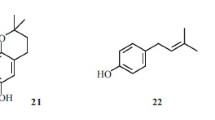
Alkylation of phenol by myrtenol in the presence of aluminum phenoxide and aluminum isopropoxide was studied in the temperature range 120–160°C. The reaction occurred with the formation of an array of alkylated phenols. Isomerization of the terpene substituent as a result of rearrangements of the bicyclic myrtenol structure was observed. The side reaction of myrtenol reduction occurred during the alkylation in the presence of aluminum isopropoxide. A significant number of compounds with two aromatic moieties was formed in the presence of aluminum phenoxide.


Similar content being viewed by others
References
S. Houry, R. Mechoulam, P. J. Fowler, E. Macko, and B. Love, J. Med. Chem., 17, No. 3, 287 (1974).
S. Houry, R. Mechoulam, and B. Love, J. Med. Chem., 18, No. 9, 951 (1975).
H. Z. Shoshan, WO Pat. No. 95/13059 (1995).
V. V. Plemenkov, Chemistry of Isoprenoids [in Russian], Izd. Altaiskogo Univ., Kaliningrad-Kazan-Barnaul, 2007.
L. A. Kheifits and I. S. Akul′chenko, in: Chemistry and Technology of Fragrances and Essential Oils [in Russian], Pishchevaya Promst., Moscow, 1968, Vol. 8, p. 142.
I. Yu. Chukicheva, A. A. Koroleva, I. V. Timusheva, and A. V. Kuchin, Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol., 52, No. 1, 27 (2009).
I. Yu. Chukicheva and A. V. Kuchin, RF Pat. No. 2003106390, 2003.
I. Yu. Chukicheva and A. V. Kuchin, Ross. Khim. Zh., 48, No. 3, 21 (2004).
V. V. Plemenkov, Introduction to the Chemistry of Natural Compounds [in Russian], Kazan, 2001.
E. A. Klein and L. Fla, US Pat. No. 2,821,547, 1953.
R. Muneyuki, Y. Yoshimura, K. Tori, Y. Terui, and J. N. Shoolery, J. Org. Chem., 53, 358 (1988).
J. J. Li, Name Reactions: A Collection of Detailed Reaction Mechanisms, Springer, Berlin, New York, 2006.
J. March, Organic Chemistry: Reactions, Mechanisms, and Structure, Wiley, New York, 1985.
J. C. Schmidhauser, G. L. Bryant, Jr., P. E. Donahue, M. F. Garbauskas, and E. A. Williams, J. Org. Chem., 60, 3612 (1995).
R. B. Bates and V. P. Thalacker, J. Org. Chem., 33, 1730 (1968).
F. Kaplan, C. O. Schulz, D. Weisledger, and C. T. Klopfenstein, J. Org. Chem., 33, 1728 (1968).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 4, July–August, 2011, pp. 494–501.
Rights and permissions
About this article
Cite this article
Koroleva, A.A., Chukicheva, I.Y., Fedorova, I.V. et al. Alkylation of phenol by myrtenol. Chem Nat Compd 47, 556 (2011). https://doi.org/10.1007/s10600-011-9995-0
Received:
Published:
DOI: https://doi.org/10.1007/s10600-011-9995-0