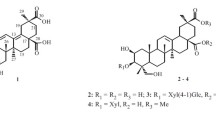
A new acylated tritrepene, 3β-hexadecanoyloxy-lup-20(29)-en-21-ol (1), along with seven known compounds, lupeol (2), betulinic acid (3), ursonic acid (4), -sitosterol (5), β-stigmasterol (6), 3-O-β-D-glucopyranosyl-β-stigmasterol (7), and palmitic acid (8), were isolated from the leaves of Rauvolfia vomitoria (Apocynaceae). Their structures were established on the basis of spectroscopic analysis and chemical evidence. The new acylated triterpene exhibited interesting antimicrobial activity against Candida albicans (a yeast) with the MIC value 64 μg/mL.

Similar content being viewed by others
References
G. N. Zirihi, L. Mambu, F. Guede-Guina, B. Bodo, and P. Grellier, J. Ethnopharmacol., 98, 281 (2005).
C. Baohui, Y. Jian, and Q. Minghua, Jingxi Huagong, 25, 37, 53 (2008).
F. Lei, G. Ting-ting, Q. Xiu-ping, and Z. Feng-sheng, Tianran Chanwu Yanjiu Yu Kaifa, 19, 113 (2007).
L. Lin, H. Hong-ping, Z. Hua, and H. Xiao-jiang, Tianran Chanwu Yanjiu Yu Kaifa, 19, 235 (2007).
D. L. Bemis, J. L. Capodice, P. Gorroochurn, A. E. Katz, and R. Buttyan, Int. J. Oncol., 29, 65 (2006).
M. Petati, F. Perlongo, T. Ruffilli, and G. F. Zini, Fitoterapia, 67, 422 (1996).
S. B. Mahato and A. P. Kundu, Phytochemistry, 37, 1517 (1994).
S. S. Awanchiri, H. T. V. Dufat, C. J. Shirri, J. M. D. Dongfack, G. M. Nguenang, S. Boutefnouchet, Z. T. Fomum, E. Seguin, P. Verite, F. Tillequin, and J. Wandji, Phytochemistry, 70, 419 (2009).
R. K. Pettit, C. A. Weber, M. J. Kean, H. Hoffmann, G. R. Pettit, R. Tan, K. S. Franks, and M. L. Horton, Antimicrob. Agents Chemother., 49, 2612 (2005).
V. Kuete, T. A. Mbaveng, M. Tsafack,V. P. Beng, F. X. Etoa, A. E. Nkengfack, J. J. M. Meyer, and N. Lall, J. Ethnopharmacol., 115, 494 (2008).
Acknowledgment
One of the authors (J. Wandji) is grateful for a grant (No. F/2624-3 F) from the International Foundation for Science (Sweden), and to the sponsorship of the “Universite Paris Descartes, France” during his multiple research visits in the “Laboratoire de Pharmacognosie, Faculte des Sciences Biologiques et Pharmaceutiques de Paris”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 3, pp. 362–364, May–June, 2011.
Rights and permissions
About this article
Cite this article
Fannang, S.V., Kuete, V., Mbazoa, C.D. et al. A new acylated triterpene with antimicrobial activity from the leaves of Rauvolfia vomitoria . Chem Nat Compd 47, 404 (2011). https://doi.org/10.1007/s10600-011-9944-y
Received:
Published:
DOI: https://doi.org/10.1007/s10600-011-9944-y