Reduced derivatives of 2,3-seco-28-oxo-19β,28-epoxy-18α-olean-2,3-dicarboxylic acid and its cyclic anhydride were prepared. Reduction of the starting 2,3-secodicarboxylic acid by NaBH4–I2 produced the 2,3-seco-2,3-dihydroxy derivative. Reaction of the starting anhydride with LiAlH4 gave the 2,3-seco2,3,19β,28-tetrahydroxy derivative. Cyclization using acidic reagents of the 2,3-seco-2,3-hydroxy- and 2,3seco-2,3,19β,28-tetrahydroxy derivatives gave the corresponding cyclic ethers containing an oxepane ring. The anhydride ring was reduced by NaBH4 to the corresponding ε-lactone, the structure of which was confirmed by an x-ray crystal structure.

Similar content being viewed by others
Notes
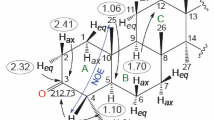
The PMR spectrum of 9 recorded in DMSO-d6 also showed resonances for OH groups as a broad triplet at 3.98 (J ~ 4, 28-OH) and a broad doublet at 4.15 (J = 4.5, 19-OH).
References
W. J. Baas, Phytochemistry, 24, 1875 (1985).
Y. Wei, C.-M. Maa, and M. Hattori, Eur. J. Med. Chem., 44, 4112 (2009).
M. Urban, J. Sarek, J. Klinot, G. Korinkova, and M. Hajduch, J. Nat. Prod., 67, 1100 (2004).
M. Urban J. Sarek, I. Tislerova, P. Dzubak, and M. Hajduch, Bioorg. Med. Chem., 13, 5527 (2005).
C. Moiteiro, F. Justino, R. Tavares, M. J. Marcelo-Curto, M. H. Florencio, M. S. J. Nascimento, M. Pedro, F. Cerqueira, and M. M. M. Pinto, J. Nat. Prod., 64, 1273 (2001).
L. Ruzicka, G. P. Frame, H. M. Leicester, M. Liguori, and H. Brungger, Helv. Chim. Acta, 17, 426 (1934).
J. Seyden-Penne, Reductions. The Alumino- and Borohydrides in Organic Synthesis, Wiley-VCH, New York, 1997, p. 92.
D. M. Bailey and R. E. Johnson, J. Org. Chem., 35, 10, 3574 (1970).
S. Brewis, T. G. Halsall, H. R. Harrison, and O. J. R. Hodder, J. Chem. Soc. D: Chem. Commun., 891 (1970).
G. A. Molander, J. B. Etter, L. S. Harring, and P.-J. Thorel, J. Am. Chem. Soc., 113, 8036 (1991).
M. Periasamy and M. Thirumalaikumar, J. Organomet. Chem., 609, 137 (2000).
J. V. B. Kanth and M. Periasamy, J. Org. Chem., 56, 5964 (1991).
M. Tudge, H. Mashima, C. Savarin, G. Humphrey, and I. Davies, Tetrahedron Lett., 49, 1041 (2008).
A. R. Katritzky and C. W. Rees, Comprehensive Heterocyclic Chemistry, Elsevier, 1997, 7, 577.
F. Chavez, S. Subrez, and M. A. Diaz, Synth. Commun., 24, 16, 2325 (1994).
T.-S. Li, J.-X. Wang, and X.-J. Zheng, J. Chem. Soc. Perkin Trans. 1, 3957 (1998).
O. Dischendorfer and O. Polak, Monatsh. Chem., 51, 43 (1929).
A. V. Shernyukov, I. Ya. Mainagashev, D. V. Korchagina, Yu. V. Gatilov, N. F. Salakhutdinov, and G. A. Tolstikov, Dokl. Akad. Nauk, 429, 3, 339 (2009);
A. V. Shernyukov, I. Ya. Mainagashev, D. V. Korchagina, Yu. V. Gatilov, N. F. Salakhutdinov, and G. A. Tolstikov, Dokl. Chem., 429, 286 (2009).
G. M. Sheldrick, SHELX-97. Programs for Crystal Structure Analysis (Release 97–2), University of Gottingen, Germany, 1997.
Acknowledgment
The work was supported financially by the RAS Presidium Basic Research Program “Development of Methods for Preparing Chemical Compounds and Creating New Materials”.
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 2, pp. 218–223, March–April, 2011
Rights and permissions
About this article
Cite this article
Shernyukov, A.V., Mainagashev, I.Y., Korchagina, D.V. et al. Reduction of 2,3-Seco-28-oxo-19β,28-epoxy-18α-olean-2,3-dicarboxylic acid and its cyclic anhydride. Chem Nat Compd 47, 237–242 (2011). https://doi.org/10.1007/s10600-011-9891-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-011-9891-7