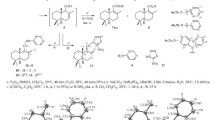
An excess of periodic acid was reacted with the diterpenoid alkaloid demethyleneldelidine (1) to produce a γ-lactone (2) in which the carbonyl at the C6-position was selectively reduced by an excess of NaBH4. The conformation of the rings, configuration of asymmetric and spiro centers, and the nature of intramolecular H-bonds in the diterpenoid alkaloid demethyleneldelidine and its reduction product 3 were analyzed by an x-ray structure analysis. The spiro center at C10 in 3 had the S-configuration. The other asymmetric centers retained the absolute configuration characteristic of alkaloids with the lycoctonine carbon skeleton. The pharmacological properties did not change significantly despite the large difference in the carbon skeletons of γ-lactone 3 and starting 1.

Similar content being viewed by others
References
F. N. Dzhakhangirov, M. N. Sultankhodzhaev, B. Tashkhodzhaev, and B. T. Salimov, Khim. Prir. Soedin., 254 (1997).
B. T. Salimov, Zh. Kh. Kuzibaeva, and F. N. Dzhakhangirov, Khim. Prir. Soedin., 384 (1996).
M. Carmack, D. W. Mayo, and J. P. Ferris, J. Am. Chem. Soc., 81, 4110 (1959).
A. D. Kuzovkov and T. F. Platonova, Zh. Obshch. Khim., 29, 2782 (1959).
B. T. Salimov, M. S. Yunusov, and S. Yu. Yunusov, Khim. Prir. Soedin., 128 (1977).
R. Shakirov, M. V. Telezhenetskaya, I. A. Bessonova, S. F. Aripova, I. A. Israilov, M. N. Sultankhodzhaev, V. I. Vinogradova, V. I. Akhmedzhanova, T. S. Tulyaganov, B. T. Salimov, and V. A. Tel’nov, Khim. Prir. Soedin., 410 (1996).
H. Boit, Ergebnisse der Alkaloid-Chemie bis 1960, Akademie-Verlag, Berlin, 1961, p. 851.
S. W. Pelletier and L. H. Keith, in: The Alkaloids. Chemistry and Physiology, R. H. F. Manske (ed.), Academic Press, New York-London, 12, 1970, p. 2.
G. M. Sheldrick, SHELXTL-Plus, Release 5.0, Siemens Analytical Instruments Inc., Madison, Wisconsin, USA, 1994.
B. T. Salimov, K. K. Turgunov, B. Tashkhodzhaev, and F. N. Dzhakhangirov, Khim. Prir. Soedin., 129 (2004).
V. V. Gatsura, Methods of Primary Pharmacological Study of Biologically Active Compounds [in Russian], Meditsina, Moscow, 1974.
Acknowledgment
The work was financed by the Basic Research Program of the CCDST, RU, Grants FA-F3-T045, FA-F3-T147, and MR-35.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 5, pp. 575–579, September–October, 2009.
Rights and permissions
About this article
Cite this article
Salimov, B.T., Tashkhodzhaev, B. & Dzhakhangirov, F.N. Structure and biologicalactivity of demethyleneldelidine and its dihydro-γ-lactone. Chem Nat Compd 45, 685–689 (2009). https://doi.org/10.1007/s10600-009-9431-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-009-9431-x