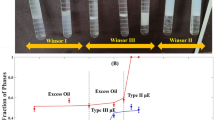
Abstract
Recent developments in predicting microemulsion phase behavior for use in chemical flooding are based on the hydrophilic-lipophilic deviation (HLD) and net-average curvature (NAC) equation-of-state (EoS). The most advanced version of the HLD-NAC EoS assumes that the three-phase micelle characteristic length is constant as parameters like salinity and temperature vary. In this paper, we relax this assumption to improve the accuracy and thermodynamic consistency of these flash calculations. We introduce a variable characteristic length in the three-phase region based on experimental data that is monotonic with salinity or other formulation variables, such as temperature and pressure. The characteristic length at the boundary of the three-phase region is then used for flash calculations in the two-phase lobes for Winsor type I/II. The functional form of the characteristic length is made consistent with the Gibbs phase rule. The improved EoS can capture asymmetric phase behavior data around the optimum, whereas current HLD-NAC based models cannot. The variable characteristic length formulation also resolves the thermodynamic inconsistency of existing phase behavior models that give multiple solutions for the optimum. We show from experimental data and theory that the inverse of the characteristic length varies linearly with formulation variables. This important result means that it is easy to predict the characteristic length in the three-phase region, which also improves the estimation of surrounding two-phase lobes. The results show that the optimum solubilization ratio can change significantly by a factor of two when variable characteristic length is included as temperature and pressure change. This can in turn greatly impact the interfacial tension (IFT) at optimum. This improved physical understanding of microemulsion phase behavior should aid in the design of surfactant blends and improve recovery predictions in a chemical flooding simulator.
Similar content being viewed by others
References
Aarra, M.G., Høiland, H., Skauge, A.: Phase behavior and salt partitioning in two-and three-phase anionic surfactant microemulsion systems: part I, phase behavior as a function of temperature. J. Colloid Interface Sci. 215(2), 201–215 (1999)
Acosta, E.J.: The HLD–NAC equation of state for microemulsions formulated with nonionic alcohol ethoxylate and alkylphenol ethoxylate surfactants. Colloids Surf. A Physicochem. Eng. Asp. 320(1), 193–204 (2008)
Acosta, E., Szekeres, E., Sabatini, D.A., Harwell, J.H.: Net-average curvature model for solubilization and supersolubilization in surfactant microemulsions. Langmuir. 19(1), 186–195 (2003)
Anklam, M.R., Prud'Homme, R.K., Warr, G.G.: Shear thinning in ternary bicontinuous and water-in-oil microemulsions. AICHE J. 41(3), 677–682 (1995)
Austad, T., Strand, S.: Chemical flooding of oil reservoirs 4. Effects of temperature and pressure on the middle phase solubilization parameters close to optimum flood conditions. Colloids Surf. A Physicochem. Eng. Asp. 108(2–3), 243–252 (1996)
Barakat, Y., Fortney, L.N., Schechter, R.S., Wade, W.H., Yiv, S.H., Graciaa, A.: Criteria for structuring surfactants to maximize solubilization of oil and water: II. Alkyl benzene sodium sulfonates. J. Colloid Interface Sci. 92(2), 561–574 (1983)
Bourrel, M., and Schechter, R. S.: Microemulsions and Related Systems: Formulation, Solvency, and Physical Properties. Editions Technip (2010)
Broens, M., Unsal, E.: Emulsification kinetics during quasi-miscible flow in dead-end pores. Adv. Water Resour. 113, 13–22 (2018)
Camilleri, D., Fil, A., Pope, G.A., Rouse, B.A., Sepehrnoori, K.: Comparison of an improved compositional micellar/polymer simulator with laboratory core floods. SPE Reserv. Eng. 2(04), 441–451 (1987)
Chou, S.I., Bae, J.H.: Phase-behavior correlation for high-salinity surfactant formulations. SPE Reserv. Eng. 3(03), 778–790 (1988)
Davis, H.T.: Factors determining emulsion type: Hydrophile—lipophile balance and beyond. Colloids Surf. A Physicochem. Eng. Asp. 91, 9–24 (1994)
De Araujo Cavalcante Filho, J.S., Delshad, M., Sepehrnoori, K.: Estimation of foam–flow parameters for local equilibrium methods by use of steady-state flow experiments and optimization algorithms. SPE Reserv. Eval. Eng. 21(01), 160–173 (2018)
De Gennes, P.G., Taupin, C.: Microemulsions and the flexibility of oil/water interfaces. J. Phys. Chem. 86(13), 2294–2304 (1982)
Dria, M.A., Schechter, R.S., Lake, L.W.: An analysis of reservoir chemical treatments. SPE Prod. Eng. 3(01), 52–62 (1988)
Ghosh, S., Johns, R.T.: An equation-of-state model to predict surfactant/oil/brine-phase behavior. SPE J. 21(04), 1–106 (2016a)
Ghosh, S., Johns, R.T.: Dimensionless equation of state to predict microemulsion phase behavior. Langmuir. 32(35), 8969–8979 (2016b)
Griffin, W.C.: Classification of surface-active agents by ‘HLB’. J. Soc. Cosmet. Chem. 1(5), 311–326 (1949)
Hand, D.B.: Dineric distribution. J. Phys. Chem. 34(9), 1961–2000 (1930)
Healy, R.N., Reed, R.L., Stenmark, D.G.: Multiphase microemulsion systems. Soc. Pet. Eng. J. 16(03), 147–160 (1976)
Huh, C.: Interfacial tensions and solubilizing ability of a microemulsion phase that coexists with oil and brine. J. Colloid Interface Sci. 71(2), 408–426 (1979)
Jennings, A.A., Kirkner, D.J.: Instantaneous equilibrium approximation analysis. J. Hydraul. Eng. 110(12), 1700–1717 (1984)
Khorsandi, S., Johns, R.T.: Robust flash calculation algorithm for microemulsion phase behavior. J. Surfactant Deterg. 19(6), 1273–1287 (2016)
Khorsandi, S., and Johns, R. T.: Extension of HLD-NAC Flash Calculation Algorithm to Multicomponent Mixtures of Microemulsion and Excess Phases. Paper presented at the SPE Improved Oil Recovery Conference, Tulsa, Oklahoma, USA (2018). https://doi.org/10.2118/190207-MS
Kilpatrick, P.K., Scriven, L.E., Davis, H.T.: Thermodynamic modeling of quaternary systems: oil/brine/surfactant/alcohol. Soc. Pet. Eng. J. 25(03), 330–342 (1985)
Krishnan, K., Chapman, B., Bates, F.S., Lodge, T.P., Almdal, K., Burghardt, W.R.: Effects of shear flow on a polymeric bicontinuous microemulsion: equilibrium and steady state behavior. J. Rheol. 46(2), 529–554 (2002)
Magzymov, D., Purswani, P., Karpyn, Z.T., Johns, R.T.: Modeling the effect of reaction kinetics and dispersion during low-salinity Waterflooding. SPE J. 26(05), 3075–3093 (2021)
Magzymov, D., Qiao, C., and Johns, R. T.: Impact of Surfactant Mixtures on Microemulsion Phase Behavior. Paper presented at the SPE Annual Technical Conference and Exhibition, Dubai, UAE (2016). https://doi.org/10.2118/181651-MS
Pope, G.A., Nelson, R.C.: A chemical flooding compositional simulator. Soc. Pet. Eng. J. 18(05), 339–354 (1978)
Rosen, M. J., Kunjappu, J.T: Surfactants and Interfacial Phenomena. John Wiley & Sons (2012)
Roshanfekr, M.: Effect of Pressure and Methane on Microemulsion Phase Behavior and its Impact on Surfactant-Polymer Flood Oil Recovery (University of Texas at Austin, Doctoral dissertation), http://hdl.handle.net/2152/ETD-UT-2010-12-2548 (2010)
Roshanfekr, M., Johns, R.T.: Prediction of optimum salinity and solubilization ratio for microemulsion phase behavior with live crude at reservoir pressure. Fluid Phase Equilib. 304(1–2), 52–60 (2011)
Rossen, W.R., Brown, R.G., Davis, H.T., Prager, S., Scriven, L.E.: Thermodynamic modeling of pseudoternary phase behavior. Soc. Pet. Eng. J. 22(06), 945–961 (1982)
Rubinstein, M., Colby, R.H.: Polymer Physics (Vol. 23). Oxford university press, New York (2003)
Salager, J.L., Marquez, N., Graciaa, A., Lachaise, J.: Partitioning of ethoxylated octylphenol surfactants in microemulsion− oil− water systems: influence of temperature and relation between partitioning coefficient and physicochemical formulation. Langmuir. 16(13), 5534–5539 (2000)
Salager, J.L., Morgan, J.C., Schechter, R.S., Wade, W.H., Vasquez, E.: Optimum formulation of surfactant/water/oil systems for minimum interfacial tension or phase behavior. Soc. Pet. Eng. J. 19(02), 107–115 (1979)
Sandler, S. I.: Chemical, Biochemical, and Engineering Thermodynamics. John Wiley and Sons (2017)
Skauge, A., Fotland, P.: Effect of pressure and temperature on the phase behavior of microemulsions. SPE Reserv. Eng. 5(04), 601–608 (1990)
Somasundaran, P., Hanna, H.S.: Adsorption of sulfonates on reservoir rocks. Soc. Pet. Eng. J. 19(04), 221–232 (1979)
Strey, R.: Microemulsion microstructure and interfacial curvature. Colloid Polym. Sci. 272(8), 1005–1019 (1994)
Trogus, F.J., Schechter, R.S., Pope, G.A., Wade, W.H.: Adsorption of mixed surfactant systems. J. Pet. Technol. 31(06), 769–778 (1979)
Unsal, E., Broens, M., Armstrong, R.T.: Pore scale dynamics of microemulsion formation. Langmuir. 32(28), 7096–7108 (2016)
Unsal, E., Rücker, M., Berg, S., Bartels, W.B., Bonnin, A.: Imaging of compositional gradients during in situ emulsification using X-ray micro-tomography. J. Colloid Interface Sci. 550, 159–169 (2019)
Widom, B.: Lattice model of microemulsions. J. Chem. Phys. 84(12), 6943–6954 (1986)
Winsor, P.A.: Hydrotropy, solubilisation and related emulsification processes. Trans. Faraday Soc. 44, 376–398 (1948)
Wu, W., Wang, P., Delshad, M., Wang, C., Pope, G. A., and Sharma, M. M.: Modeling Non-equilibrium Mass Transfer Effects for a Gas Condensate Field. Paper presented at the SPE Asia Pacific Conference on Integrated Modelling for Asset Management, Kuala Lumpur, Malaysia (1998). https://doi.org/10.2118/39746-MS
Acknowledgments
The authors thank the member companies of the Enhanced Oil Recovery JIP in the EMS Energy Institute, at the Pennsylvania State University for their financial support. The authors also greatly acknowledge the funding support from the Abu Dhabi National Oil Company. Dr. Russell T. Johns holds the George E. Trimble Chair in Earth and Mineral Sciences at Penn State.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Magzymov, D., Johns, R.T. Inclusion of variable characteristic length in microemulsion flash calculations. Comput Geosci 26, 995–1010 (2022). https://doi.org/10.1007/s10596-022-10158-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10596-022-10158-2