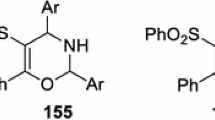
The interaction employing sulfamide and enolizable ketones was closely inspected in Biginelli reaction. The three-component variation of the reaction ''sulfamide + ketone + anisaldehyde'' and ''sulfamide + ketone + N,N-diethylacetoacetamide'' featured a lack of selectivity and provided a mixture of possible condensation products. Selectivity was improved when ketones were used in the reaction both as carbonyl and enol components. It was shown that trimethylsilyl chloride promoted interaction of sulfamide with ketones to afford 1,2,6- thiadiazines. Limitations were noted and unpredictable outcomes of the reaction were discussed as well.





Similar content being viewed by others
References
(a) Chorlton, A. P. In Ullmann, F. Ullmann's Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, 1999, 1st ed. (b) de Fátima, Â.; Braga, T. C.; Neto, L. d. S.; Terra, B. S.; Oliveira, B. G. F.; da Silva, D. L.; Modolo, L. V. J. Adv. Res. 2015, 6, 363.
(a) Chopda, L. V.; Dave, P. N. ChemistrySelect 2020, 5, 5552. (b) Shumaila, A. M. A.; Al-Thulaia, A. A. N. Synth. Commun. 2019, 49, 1613. (c) Heravi, M. M.; Moradi, R.; Mohammadkhani, L.; Moradi, B. Mol. Diversity 2018, 22, 751.
(a) Mayer, T. U.; Kapoor, T. M.; Haggarty, S. J.; King, R. W.; Schreiber, S. L.; Mitchison, T. J. Science 1999, 286(5441), 971. (b) Kaan, H. Y. K.; Ulaganathan, V.; Rath, O.; Prokopcová, H.; Dallinger, D.; Kappe, C. O.; Kozielski, F. J. Med. Chem. 2010, 53, 5676. (c) Prokopcová, H.; Dallinger, D.; Uray, G.; Kaan, H. Y. K.; Ulaganathan, V.; Kozielski, F.; Laggner, C.; Kappe, C. O. ChemMedChem 2010, 5, 1760.
Gong, L.-Z.; Chen, X.-H.; Xu, X.-Y. Chem.–Eur. J. 2007, 13, 8920.
Barrow, J. C.; Nantermet, P. G.; Selnick, H. G.; Glass, K. L.; Rittle, K. E.; Gilbert, K. F.; Steele, T. G.; Homnick, C. F.; Freidinger, R. M.; Ransom, R. W.; Kling, P.; Reiss, D.; Broten, T. P.; Schorn, T. W.; Chang, R. S. L.; O'Malley, S. S.; Olah, T. V.; Ellis, J. D.; Barrish, A.; Kassahun, K.; Leppert, P.; Nagarathnam, D.; Forray, C. J. Med. Chem. 2000, 43, 2703.
Atwal, K. S.; Swanson, B. N.; Unger, S. E.; Floyd, D. M.; Moreland, S.; Hedberg, A.; O'Reilly, B. C. J. Med. Chem. 1991, 34, 806.
(a) Sánchez-Sancho, F.; Escolano, M.; Gaviña, D.; Csáky, A. G.; Sánchez-Roselló, M.; Díaz-Oltra, S.; del Pozo, C. Pharmaceuticals 2022, 15, 948. (b) Kaur, R.; Chaudhary, S.; Kumar, K.; Gupta, M. K.; Rawal, R. K. Eur. J. Med. Chem. 2017, 132, 108. (c) Marinescu, M. Molecules 2021, 26, 6022.
Lacotte, P.; Buisson, D.-A.; Ambroise, Y. Eur. J. Med. Chem. 2013, 62, 722.
Atwal, K. S.; Rovnyak, G. C.; O'Reilly, B. C.; Schwartz, J. J. Org. Chem. 1989, 54, 5898.
Ahmad, M. J.; Hassan, S. F.; Nisa, R. U.; Ayub, K.; Nadeem, M. S.; Nazir, S.; Ansari, F. L.; Qureshi, N. A.; Rashid, U. Med. Chem. Res. 2016, 25, 1877.
Devineni, S. R.; Madduri, T. R.; Chamarthi, N. R.; Liu, C.-Q.; Pavuluri, C. M. Chem. Heterocycl. Compd. 2019, 55, 266. 12. Ninomiya, M.; Garud, D. R.; Koketsu, M. Heterocycles 2010, 81, 2027.
(a) Peixoto, C.; Laurin, P.; Klich, M.; Dupuis-Hamelin, C.; Mauvais, P.; Lassaigne, P.; Bonnefoy, A.; Musicki, B. Tetrahedron Lett. 2000, 41, 1741. (b) Ukrainets, I. V.; Petrushova, L. A.; Dzyubenko, S. P. Chem. Heterocycl. Compd. 2013, 49, 1378. (c) Grygoriv, G. V.; Lega, D. A.; Zaprutko, L.; Gzella, A. K.; Wieczorek-Dziurla, E.; Chernykh, V. P.; Shemchuk, L. A. Chem. Heterocycl. Compd. 2019, 55, 254.
14. Alkorta, I.; Goya, P.; Páez, J. A.; Pfleiderer, W. Pteridines 1990, 2, 3.
15. Cano, C.; Goya, P.; Páez, J. A.; Girón, R.; Sánchez, E.; Martín, M. I. Bioorg. Med. Chem. 2007, 15, 7480.
16. Esteban, A. I.; Juanes, O.; Conde, S.; Goya, P.; De Clercq, E.; Martínez, A. Bioorg. Med. Chem. 1995, 3, 1527.
17. Guerra, A.; Gonzalez-Naranjo, P.; Campillo, N. E.; Varela, J.; Lavaggi, M. L.; Merlino, A.; Cerecetto, H.; González, M.; Gomez-Barrio, A.; Escario, J. A.; Fonseca-Berzal, C.; Yaluf, G.; Paniagua-Solis, J.; Páez, J. A. Eur. J. Med. Chem. 2017, 136, 223.
18. Esteban, A. I.; De Clercq, E.; Martinez, A. Nucleosides, Nucleotides Nucleic Acids 1997, 16, 265.
19. Goya, P.; Nieves, R.; Ochoa, C.; Rodellas, C.; Martinez-Ripoll, M.; García-Blanco, S. Can. J. Chem. 1987, 65, 298.
20. Fensome, A.; Goldberg, J.; McComas, C. C.; Trybulski, E. J.; Woodworth, R. P.; Deecher, D. C.; Whiteside, G. T.; Zhang, P. Bioorg. Med. Chem. Lett. 2010, 20, 1555.
21. Huber, R.; Otto, S. In Reviews of Environmental Contamination and Toxicology: Continuation of Residue Reviews; Ware, G. W., Ed.; Springer New York: New York, 1994, p. 111.
22. Castro, A.; Martínez, A.; Cardelús, I.; Llenas, J. Bioorg. Med. Chem. 1995, 3, 179.
23. Zimmermann, R. Angew. Chem. 1963, 75, 1025.
(a) Passia, M. T.; Schöbel, J.-H.; Lentelink, N. J.; Truong, K.-N.; Rissanen, K.; Bolm, C. Org. Biomol. Chem. 2021, 19, 9470. (b) Schöbel, J.-H.; Passia, M. T.; Wolter, N. A.; Puttreddy, R.; Rissanen, K.; Bolm, C. Org. Lett. 2020, 22, 2702. (c) Schöbel, J.-H.; Liang, W.; Wöll, D.; Bolm, C. J. Org. Chem. 2020, 85, 15760.
(a) Albericio, F.; Bryman, L. M.; Garcia, J.; Michelotti, E. L.; Nicolás, E.; Tice, C. M. J. Comb. Chem. 2001, 3, 290. (b) Campbell, A. D.; Birch, A. M. Synlett 2005, 834.
26. Johnson, P. D.; Jewell, S. A.; Romero, D. L. Tetrahedron Lett. 2003, 44, 5483.
Weintraub, P. M. In Comprehensive Heterocyclic Chemistry III; Katritzky, A. R.; Ramsden, C. A.; Scriven, E. F. V.; Taylor, R. J. K., Eds.; Elsevier: Oxford, 2008, p. 355.
28. Ouchi, A.; Moeller, T. J. Org. Chem. 1964, 29, 1865.
29. Knollmüller, M.; Reich, K. R. Monatsh. Chem. 1975, 106, 1095.
30. Lee, C.-H.; Kohn, H. J. Heterocycl. Chem. 1990, 27, 2107.
31. Terrab, L.; Rosenker, C. J.; Johnstone, L.; Ngo, L. K.; Zhang, L.; Ware, N. F.; Miller, B.; Topacio, A. Z.; Sannino, S.; Brodsky, J. L.; Wipf, P. ACS Med. Chem. Lett. 2020, 11, 984.
Wipf, P.; Ngo, L. К.; Terraв, L.; Brodsky, J. L. WO Patent 2021/163513 A1.
33. Bouzina, A.; Berredjem, M.; Belhani, B.; Bouacida, S.; Marminon, C.; Le Borgne, M.; Bouaziz, Z.; Aissaoui, M. Res. Chem. Intermed. 2021, 47, 1359.
34. Krauskopf, F.; Truong, K.-N.; Rissanen, K.; Bolm, C. Org. Lett. 2021, 23, 2699.
(a) Ryabukhin, S. V.; Plaskon, A. S.; Boron, S. Y.; Volochnyuk, D. M.; Tolmachev, A. A. Mol. Diversity 2011, 15, 189. (b) Ryabukhin, S. V.; Plaskon, A. S.; Ostapchuk, E. N.; Volochnyuk, D. M.; Tolmachev, A. A. Synthesis 2007, 417. (c) Ryabukhin, S. V.; Plaskon, A. S.; Bondarenko, S. S.; Ostapchuk, E. N.; Grygorenko, O. O.; Shishkin, O. V.; Tolmachev, A. A. Tetrahedron Lett. 2010, 51, 4229. (d) Ryabukhin, S. V.; Naumchik, V. S.; Grygorenko, O. O.; Tolmachev, A. A. J. Heterocycl. Chem. 2012, 49, 1147. (e) Ostapchuk, E. N.; Plaskon, A. S.; Grygorenko, O. O.; Tolmachev, A. A.; Ryabukhin, S. V. J. Heterocycl. Chem. 2013, 50, 1299.
(a) Volochnyuk, D. M.; Ryabukhin, S. V.; Plaskon, A. S.; Grygorenko, O. O. Synthesis 2009, 3719. (b) Mityuk, A. P.; Kiriakov, O. M.; Tiutiunnyk, V. V.; Lebed, P. S.; Grabchuk, G. P.; Rusanov, E. B.; Volochnyuk, D. M.; Ryabukhin, S. V. J. Org. Chem. 2023, 88, 2961. (c) Ryabukhin, S. V.; Panov, D. M.; Plaskon, A. S.; Tolmachev, A. A.; Smaliy, R. V. Monatsh. Chem. 2012, 143, 1507.
Lyapunov, A. Yu.; Tarnovskiy, A. V.; Boron, S. Yu.; Rusanov, E. B.; Grabchuk, G. P.; Volochnyuk, D. M.; Ryabukhin S. V. ChemRxiv https://doi.org/10.26434/chemrxiv-2023-j5hbc.
38. Li, N.; Chen, X.-H.; Song, J.; Luo, S.-W.; Fan, W.; Gong, L.-Z. J. Am. Chem. Soc. 2009, 131, 15301.
39. Nishimura, Y.; Kikuchi, H.; Kubo, T.; Arai, R.; Toguchi, Y.; Yuan, B.; Sunaga, K.; Cho, H. Chem. Pharm. Bull. 2022, 70, 111.
Neogi, P. J. Chem. Soc., Trans. 1911, 99, 1249.
41. Dressler, J. J.; Miller, S. A.; Meeuwsen, B. T.; Riel, A. M. S.; Dahl, B. J. Tetrahedron 2015, 71, 283.
42. Bartlett, P. D.; Roha, M.; Stiles, R. M. J. Am. Chem. Soc. 1954, 76, 2349.
(a) Lee, C. H.; Kohn, H. J. Org. Chem. 1990, 55, 6098. (b) Lee, C. H.; Kohn, H. Heterocycles 1988, 27, 2581.
(a) Dusemund, J.; Schurreit, T. Arch. Pharm. 1987, 320, 534. (b) Dusemund, J.; Schurreit, T. Arch. Pharm. 1986, 319, 826. (c) Dusemund, J. Arch. Pharm. 1977, 310, 417. (d) Dusemund, J. Arch. Pharm. 1977, 310, 404. (e) Dusemund, J. Arch. Pharm. 1974, 307, 881.
Sheldrick, G. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2023, 59(6/7), 500–507
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lyapunov, A.Y., Tarnovskiy, A.V., Osokina, M.H. et al. Sulfo-Biginelli reaction: an insight into interaction between sulfamides and enolizable ketones. Chem Heterocycl Comp 59, 500–507 (2023). https://doi.org/10.1007/s10593-023-03222-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-023-03222-x