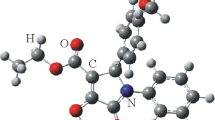
A novel methodology has been developed to afford 1,4,5-trisubstituted 1-benzyl-5-formyl-1,2,3-triazole-4-carboxylates in one step under mild conditions. This method includes the reduction of the ester functional group to an aldehyde using lithium tri-tert-butoxyaluminum hydride. Especially, this method offers a different perspective for fully substituted 1,2,3-triazoles with high regioselectivity and high yields. The structure of fully substituted triazoles was verified using FTIR, 1H, 13C NMR spectroscopy, HRMS, advanced NMR techniques (COSY, C-APT, HSQC, and HMBC), and X-ray crystallography.




Similar content being viewed by others
References
Buckle, D. R.; Rockell, C. J. M; Smith, H.; Spicer, B. A. J. Med. Chem. 1986, 29, 2262.
Thirumurugan, P.; Matosiuk, D.; Jozwiak, K. Chem. Rev. 2013, 113, 4905.
Lauria, A.; Delisi, R.; Mingoia, F.; Terenzi, A.; Martorana, A.; Barone, G.; Almerico, A. M. Eur. J. Org. Chem. 2014, 3289.
Nguyen, D. T.; Ngo, T. H.; Tran, H. T.; Dinh, T. P.; Do, P. T.; Nguyen, H. B.; Tran, L. T. P.; Ta, H. M. Chem. Heterocycl. Compd. 2021, 57, 1037.
Bozorov, K.; Zhao, J.; Aisa, H. A. Bioorg. Med. Chem. 2019, 27, 3511.
Tapkir, S. R.; Patil, R. H.; Galave, S. A.; Phadtare, G. R.; Khedkar, V. M.; Garud, D. R. J. Heterocycl. Chem. 2022, 59, 739.
Oubella, A.; Bimoussa, A.; Byadi, S.; Fawzi, M.; Laamari, Y.; Auhmani, A.; Morjani, H.; Robert, A.; Riahi, A.; Itto, M. Y. A. J. Mol. Struct. 2022, 1265, 133383.
Huisgen, R. Angew. Chem., Int. Ed. 1963, 2, 565.
Dheer, D.; Singh, V.; Shankar, R. Bioorg. Chem. 2017, 71, 30.
Kolb, H. C.; Finn, M. G.; Sharpless, K. B. Angew. Chem., Int. Ed. 2001, 40, 2004.
Boren, B. C.; Narayan, S.; Rasmussen, L. K.; Zhang, L.; Zhao, H.; Lin, Z.; Jia, G.; Fokin, V. V. J. Am. Chem. Soc. 2008, 130, 8923.
Li, L.; Shang, T.; Ma, X.; Guo, H.; Zhu, A.; Zhang, G. Synlett 2015, 26, 695.
Chuprakov, S.; Chernyak, N.; Dudnik A. S.; Gevorgyan, V. Org. Lett. 2007, 9, 2333.
Shiri, P.; Amani, A. M.; Mayer-Gall, T. Beilstein J. Org. Chem. 2021, 17, 1600.
Ramanaiah, K. C. V.; Stevens, E. D.; Trudell, M. L.; Pagoria, P. F. J. Heterocycl. Chem. 2000, 37, 1597.
Pokhodylo, N. T.; Shyyka, O. Ya.; Obushak, M. D. Chem. Heterocycl. Compd. 2018, 54, 773.
Fariña, F.; Fernández, P.; Fraile, M. T.; Martín, M. V.; Martín, M. R. Heterocycles 1989, 29, 967.
Baykal, A.; Zhang, D.; Knelles, J.; Alt, I. T.; Plietker, B. Asian J. Chem. 2019, 14, 3003.
Jomova, K.; Valko, M. Toxicology 2011, 283, 65.
Gierlich, J.; Burley, G. A.; Gramlich, P. M. E.; Hammond, D. M.; Carell, T. Org. Lett. 2006, 8, 3639.
Kennedy, D. C.; McKay, C. S.; Legault, M. C. B.; Danielson, D. C.; Blake, J. A.; Pegoraro, A. F.; Stolow, A.; Mester, Z.; Pezacki, J. P. J. Am. Chem. Soc. 2011, 133, 17993.
Zheng, H.; McDonald, R.; Hall, D. G. Chem.–Eur. J. 2010, 16, 5454.
Carey, F. A.; Sundberg, R. J. Advanced Organic Chemistry: Part B: Reaction and Synthesis; Springer, 2007.
Pérez-Serrano, L.; Casarrubios, L.; Dominguez, G.; González-Pérez, P.; Pérez-Castells, J. Synthesis 2002, 1810.
Dransfield, P. J.; Dilley, A. S.; Wang, S.; Romo, D. Tetrahedron 2006, 62, 5223.
Devine, W. G.; Diaz-Gonzalez, R.; Ceballos-Perez, G.; Rojas, D.; Satoh, T.; Tear, W.; Ranade, R. M.; Barros-Álvarez, X.; Hol, W. G. J.; Buckner, F. S.; Navarro, M.; Pollastri, M. P. ACS Infect. Dis. 2017, 3, 225.
Lopchuk, J. M.; Gribble, G. W. Heterocycles 2011, 82, 1617.
Deng, Y.; Liang, X.; Wei, K.; Yang, Y. R. J. Am. Chem. Soc. 2021, 143, 20622.
Yoshida, K.; Hayashi, K.; Yanagisawa, A. Org. Lett. 2011, 13, 4762.
Yasui, E.; Tsuda, J.; Ohnuki, S.; Nugumo, S. Chem. Pharm. Bull. 2016, 64, 1262.
Shanmugavelan, P.; Nagarajan, S.; Sathishkumar, M.; Ponnuswamy, A.; Yogeeswari, P.; Sriram, D. Bioorg. Med. Chem. Lett. 2011, 21, 7273.
Shaikh, M. H.; Subhedar, D. D.; Arkile, M.; Khedkar, V. M.; Jadhav, N.; Sarkar, D.; Shingate, B. B. Bioorg. Med. Chem. Lett. 2016, 26, 561.
Sri Ramya, P. V.; Angapelly, S.; Guntuku, L.; Digwal, C. S.; Babu, B. N.; Naidu, V. G. M.; Kamal, A. Eur. J. Med. Chem. 2017, 127, 100.
Rodríguez-Hernández, D.; Demuner, A. J.; Barbosa, L. C. A.; Heller, L.; Csuk, R. Eur. J. Med. Chem. 2016, 115, 257.
Biagi, G.; Giorgi, I.; Livi, O.; Manera, C.; Scartoni, V.; Betti, L.; Giannaccini, G.; Lucacchini, A. Farmaco 1999, 54, 615.
Abu-Orabi, S. T.; Atfah, A. M.; Jibril, I.; Mari'i, F. M.; Ali, A. A. J. Heterocycl. Chem. 1989, 26, 1461.
Barman, M. K.; Sinha, A. K.; Nembenna, S. Green Chem. 2016, 18, 2534.
Tarawneh, A. H.; Al-Momani, L. A.; León, F.; Jain, S. K.; Gadetskaya, V. A.; Abu-Orabi, S. T.; Tekwani, B. L.; Cutler, S. J. Med. Chem. Res. 2018, 27, 1269.
Butler, C. R.; Bendesky, J.; Schoffstall, A. M. Molecules 2012, 26, 5589.
SAINT, version 8.34A; Bruker AXS, Inc.: Madison, 2013.
SADABS, version 2014/5; Bruker AXS, Inc.: Madison, 2014.
APEX2, version 2014.11–0; Bruker AXS, Inc.: Madison, 2014.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Adv. 2015, 71, 3.
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339.
Spek, A. L. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2009, 65, 148.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2023, 59(4/5), 267–276
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Erdemir, G.Y., Altundas, A. Highly regioselective one-step synthesis of 1-benzyl-5-formyl-1,2,3-triazole-4-carboxylates. Chem Heterocycl Comp 59, 267–276 (2023). https://doi.org/10.1007/s10593-023-03192-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-023-03192-0