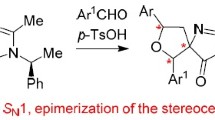
Interaction of imidazol-5-one-based donor–acceptor cyclopropanes with thiourea and thioacetamide was studied. Previously undescribed spirocyclic 2-amino- and 2-iminotetrahydrothiophenes are formed in the reaction.

Similar content being viewed by others
References
(a) Reissig, H.-U.; Zimmer, R. Chem. Rev. 2003, 103, 1151. (b) Schneider, T. F.; Kaschel, J.; Werz, D. B. Angew. Chem., Int. Ed. 2014, 53, 5504.
(a) England, D. B.; Kuss, T. D. O.; Keddy, R. G.; Kerr, M. A. J. Org. Chem. 2001, 66, 4704. (b) de Nanteuil, F.; Waser, J. Angew. Chem., Int. Ed. 2011, 50, 12075. (c) Trost, B. M.; Morris, P. J. Angew. Chem., Int. Ed. 2011, 50, 6167. (d) Racine, S.; de Nanteuil, F.; Serrano, E.; Waser, J. Angew. Chem., Int. Ed. 2014, 53, 8484.
(a) Pohlhaus, P. D.; Johnson, J. S. J. Am. Chem. Soc. 2005, 127, 16014. (b) Pohlhaus, P. D.; Sanders, S. D.; Parsons, A. T.; Li, W.; Johnson, J. S. J. Am. Chem. Soc. 2008, 130, 8642. (c) Parsons, A. T.; Johnson, J. S. J. Am. Chem. Soc. 2009, 131, 3122.
(a) Carson, C. A.; Kerr, M. A. J. Org. Chem. 2005, 70, 8242. (b) Jackson, S. K.; Karadeolian, A.; Driega, A. B.; Kerr, M. A. J. Am. Chem. Soc. 2008, 130, 4196. (c) Parsons, A. T.; Smith, A. G.; Neel, A. J.; Johnson, J. S. J. Am. Chem. Soc. 2010, 132, 9688. (d) Preindl, J.; Chakrabarty, S.; Waser, J. Chem. Sci. 2017, 8, 7112. (e) Zhang, M.-C.; Wang, D.-C.; Xie, M.-S.; Qu, G.-R.; Guo, H.-M.; You, S.-L. Chem 2019, 5, 156.
Chakrabarty, S.; Chatterjee, I.; Wibbeling, B.; Daniliuc, C. G.; Studer, A. Angew. Chem., Int. Ed. 2014, 53, 5964.
(a) Korotkov, V. S.; Larionov, O. V.; Hofmeister, A.; Magull, J.; de Meijere, A. J. Org. Chem. 2007, 72, 7504. (b) Tomilov, Y. V.; Novikov, R. A.; Nefedov, O. M. Tetrahedron 2010, 66, 9151.
(a) Yadav, V. K.; Sriramurthy, V. Angew. Chem., Int. Ed. 2004, 43, 2669. (b) Racine, S.; Hegedüs, B.; Scopelliti, R.; Waser, J. Chem.–Eur. J. 2016, 22, 11997. (c) Pagenkopf, B. L.; Yu, M. Org. Lett. 2003, 5, 5099. (d) Yu, M.; Pagenkopf, B. L. J. Am. Chem. Soc. 2003, 125, 8122. (e) Young, I. S.; Kerr, M. A. Angew. Chem., Int. Ed. 2003, 42, 3023. (f) Sibi, M. P.; Ma, Z.; Jasperse, C. P. J. Am. Chem. Soc. 2005, 127, 5764. (g) Kang, Y.-B.; Sun, X.-L.; Tang, Y. Angew. Chem., Int. Ed. 2007, 46, 3918. (h) Xu, P.-W.; Liu, J.-K.; Shen, L.; Cao, Z.-Y.; Zhao, X.-L.; Yan, J.; Zhou, J. Nat. Commun. 2017, 8, 1619.
(a) Augustin, A. U.; Sensse, M.; Jones, P. G.; Werz, D. B. Angew. Chem., Int. Ed. 2017, 56, 14293. (b) Augustin, A. U.; Busse, M.; Jones, P. G.; Werz, D. B. Org. Lett. 2018, 20, 820. (c) Kreft, A.; Jones, P. G.; Werz, D. B. Org. Lett. 2018, 20, 2059.
Matsumoto, Y.; Nakatake, D.; Yazaki, R.; Ohshima, T. Chem.–Eur. J. 2018, 24, 6062.
Xie, M.-S.; Zhao, G.-F.; Qin, T.; Suo, Y.-B.; Qua, G.-R.; Guo, H.-M. Chem. Commun. 2019, 55, 1580.
(a) Mikhaylov, A. A.; Kuleshov, A. V.; Solyev, P. N.; Korlyukov, A. A.; Dorovatovskii, P. V.; Mineev, K. S.; Baranov, M. S. Org. Lett. 2020, 22, 2740. (b) Mikhaylov, A. A.; Solyev, P. N.; Kuleshov, A. V.; Kublitskii, V. S.; Korlyukov, A. A.; Lushpa, V. A.; Baranov, M. S. Chem. Heterocycl. Compd. 2020, 56, 1092.
Huang, Y.; Dömling, A. Mol. Diversity 2011, 15, 3.
(a) Gewald, K.; Schinke, E.; Böttcher, H. Chem. Ber. 1966, 99, 94. (b) Sabnis, R. W.; Rangnekar, D. W.; Sonawane, N. D. J. Heterocycl. Chem. 1999, 36, 333. (c) Özbek, H.; Veljkovic, I. S.; Reissig, H.-U. Synlett 2008, 3145.
Ikonnikova, V. A.; Zhigileva, E. A.; Kuleshov, A. V.; Shirokova, V. V.; Baranov, M. S.; Mikhaylov A. A. Mendeleev Commun. 2021, 31, 657.
Baldridge, A.; Kowalik, J.; Tolbert, L. M. Synthesis 2010, 2424.
Chen, K.-Y.; Cheng, Y.-M.; Lai, C.-H.; Hsu, C.-C.; Ho, M.-L.; Lee, G.-H.; Chou, P.-T. J. Am. Chem. Soc. 2007, 129, 4534.
Zaitseva, S. O.; Golodukhina, S. V.; Baleeva, N. S.; Levina, E. A.; Smirnov, A. Yu.; Zagudaylova, M. B.; Baranov, M. S. ChemistrySelect 2018, 3, 8593.
Chen, Y.-H.; Lo, W.-J.; Sung, K. J. Org. Chem. 2013, 78, 301.
APEX3, RLATT, CELL_NOW, TWINABS, SAINT-Plus, SADABS; Bruker AXS Inc.: Madison, 2016.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2015, A71, 3.
Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem. 2015, C71, 3.
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J, Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339.
The authors gratefully acknowledge support from the Russian Science Foundation, grant No. 20-73-10195.
A. A. Korlyukov acknowledges the Ministry of Science and Higher Education of the Russian Federation (Contract/agreement No. 075-00697-22-00) for support of employment of the equipment of Center for Molecular Composition Studies of A. N. Nesmeyanov Institute of Organoelement Compounds of Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2022, 58(12), 756–763
Supplementary Information
ESM 1
(PDF 5318 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nikolaev, S.E., Sokolov, A.I., Mikhaylov, A.A. et al. Imidazol-5-one-based donor–acceptor cyclopropanes in reaction with C=S dipolarophiles. Chem Heterocycl Comp 58, 756–763 (2022). https://doi.org/10.1007/s10593-023-03153-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-023-03153-7