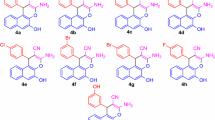
A regioselective method for the synthesis of ethyl 3,4-dihydrochromeno[3,4-c]pyrazole-1-carboxylates from 2-substituted 3-nitro-2Hchromenes and ethyl diazoacetate in 68–87% yields was developed. This approach involves an AgOAc-catalyzed 1,3-dipolar cycloaddition of ethyl diazoacetate to nitrochromenes followed by elimination of HNO2 by the action of DBU.
Similar content being viewed by others
References
(a) Kumar, V.; Kaur, K.; Gupta, G. K.; Sharma, A. K. Eur. J. Med. Chem. 2013, 69, 735. (b) Ebenezer, O.; Shapi, M.; Tuszynski, J. A. Biomedicines 2022, 10, 1124. (c) Santos, N. E.; Carreira, A. R. F.; Silva, V. L. M.; Braga, S. S. Molecules 2020, 25, 1364. (d) Ansari, A.; Ali, A.; Asif, M.; Shamsuzzaman New J. Chem. 2017, 41, 16. (e) Faisal, M.; Saeed, A.; Hussain, S.; Dar, P.; Larik, F. A. J. Chem. Sci. 2019, 131, 70. (f) Karrouchi, K.; Radi, S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y.; Al-aizari, F. A.; Ansar, M. Molecules 2018, 23, 134. (g) Mykhailiuk, P. K. Chem. Rev. 2021, 121, 1670.
(a) Halimehjani, A. Z.; Namboothiri, I. N. N.; Hooshmand, S. E. RSC Adv. 2014, 4, 48022. (b) Motornov, V. A.; Ioffe, S. L.; Tabolin, A. A. In Targets Heterocyclic Systems; Attanasi, O. A.; Spinelli, D., Eds.; Royal Society of Chemistry: Cambridge, 2019, Vol. 23, p. 237. (c) Fustero, S.; Sánchez-Roselló, M.; Barrio, P.; Simón-Fuentes, A. Chem. Rev. 2011, 111, 6984. (d) Baranski, A.; Kelarev, V. I. Chem. Heterocycl. Compd. 1990, 26, 371. (e) Shapiro, E. А.; Dyatkin, А. B.; Nefedov, О. M. Diazoefiry (Diazoesters [in Russian]); Moscow: Nauka, 1992.
(a) Korotaev, V. Yu.; Sosnovskikh, V. Ya.; Barkov, A. Yu. Russ. Chem. Rev. 2013, 82, 1081. (b) Korotaev, V. Yu.; Kutyashev, I. B.; Barkov, A. Yu.; Sosnovskikh, V. Ya. Russ. Chem. Rev. 2019, 88, 27. (c) Das, S. Synth. Commun. 2022, 52, 637.
(a) Kodukulla, R. P. K.; Hariharan, S.; Trivedi, G. K. Tetrahedron 1994, 50, 4623. (b) Muruganantham, R.; Namboothiri, I. N. N. J. Org. Chem. 2010, 75, 2197. (c) Kumar, R.; Namboothiri, I. N. N. Org. Lett. 2011, 13, 4016. (d) Chen, Z.; Zhang, Y.; Nie, J.; Ma, J.-A. Org. Lett. 2018, 20, 2120. (e) Chen, Z.; Zheng, Y.; Ma, J.-A. Angew. Chem., Int. Ed. 2017, 56, 4569. (f) Fu, X.; Li, H.; Ren, D.; Li, X. J. Chem. Res. 2017, 41, 709.
Peng, X.; Zhang, X.; Li, S.; Lu, Y.; Lan, L.; Yang, C. Org. Chem. Front. 2019, 6, 1775.
(a) Xie, J.-W.; Wang, Z.; Yang, W.-J.; Kong, L.-C.; Xu, D.-C. Org. Biomol. Chem. 2009, 7, 4352. (b) Ivanova, O. A.; Budynina, E. M.; Averina, E. B.; Kuznetsova, T. S.; Grishin, Y. K.; Zefirov, N. S. Synthesis 2007, 2009. (c) Parham, W. E.; Bleasdale, J. L. J. Am. Chem. Soc. 1950, 72, 3843.
(a) Barkov, A. Yu.; Kochnev, I. A.; Simonov, N. S.; Kutyashev, I. B.; Zimnitskiy, N. S.; Korotaev, V. Yu.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2021, 57, 1204. (b) Kutyashev, I. B.; Ulitko, M. V.; Barkov, A. Yu.; Zimnitskiy, N. S.; Korotaev, V. Yu.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2021, 57, 751. (c) Korotaev, V. Yu.; Barkovskii, S. V.; Kutyashev, I. B.; Ulitko, M. V.; Barkov, A. Yu.; Zimnitskiy, N. S.; Kochnev, I. А.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2021, 57, 679. (d) Kutyashev, I. B.; Sannikov, M. S.; Kochnev, I. A.; Barkov, A. Yu.; Zimnitskiy, N. S.; Korotaev, V. Yu.; Sosnovskikh, V. Ya. SynOpen 2021, 5, 1. (e) Korotaev, V. Yu.; Kutyashev, I. B.; Barkov, A. Yu.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2017, 53, 597.
(a) Korotaev, V. Yu.; Kutyashev, I. B.; Sosnovskikh, V. Ya. Heteroat. Chem. 2005, 16, 492. (b) Sakakibara, T.; Koezuka, M.; Sudoh, R. Bull. Chem. Soc. Jpn. 1978, 51, 3095. (c) Barkov, A. Yu.; Korotaev, V. Yu.; Kotovich, I. V.; Zimnitskiy, N. S.; Kutyashev, I. B.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2016, 52, 814.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2022, 58(11), 646–650
Supplementary Information
ESM 1
(PDF 2170 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bykova, L.S., Kochnev, I.А., Barkov, A.Y. et al. An AgOAc-catalyzed reaction of 3-nitro-2H-chromenes with ethyl diazoacetate: an efficient one-pot synthesis of ethyl 3,4-dihydrochromeno[3,4-c]pyrazole-1-carboxylates. Chem Heterocycl Comp 58, 646–650 (2022). https://doi.org/10.1007/s10593-022-03128-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-022-03128-0