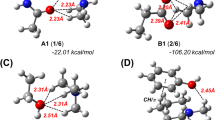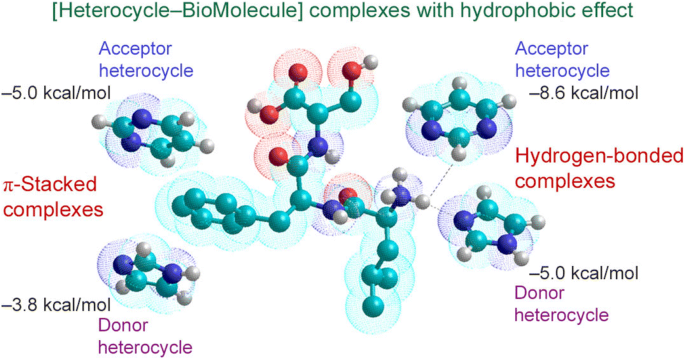
For the estimation of the biological affinity of nitrogen-containing π-conjugated heterocyclic systems toward amino acid residues in proteins, the fragment-to-fragment approach was proposed. Two mechanisms of complexation between the heterocycle molecule with different donor/acceptor properties and the amino acid residue in the active part of the protein biomolecule were considered. One of these mechanisms is the π-stacking interaction and the other is formation of hydrogen bonds with model amino acid residues. It was found that heterocycles with a π-conjugated electron-acceptor moiety form a more stable heterocycle–biomolecule complex with protein fragments. Nitrogen-containing conjugated heterocycles with several nitrogen atoms form poly-hydrogen-bonded complexes. The stabilization energy of complexes with two pyrimidine–biomolecule hydrogen bonds increases by 4–6 kcal/mol compared to similar complexes with one hydrogen bond. Hydrophobic interactions are much more sensitive to the donor-acceptor properties of heterocycles in the formation of hydrogen-bonded complexes than in the formation of π-stacked complexes. The hydrophobic effect in the fragment-to-fragment approach allows us to see the values of the stabilization energies of the heterocycle–biomolecule complexes as close as possible to the experimentally studied biological systems.






Similar content being viewed by others
References
Siwach, A.; Verma, P. K. BMC Chem. 2021, 15, 12.
Waller, D. G.; Sampson, A. P. Medical Pharmacology & Therapeutics; Elsevier: Edinburgh, 2018, p. 581.
Mohana, K. N.; Prasanna K. B. N.; Mallesha, L. Drug Invent. Today 2013, 5, 216.
Prachayasittikul, S.; Pingaew, R.; Worachartcheewan, A.; Sinthupoom, N.; Prachayasittikul, V.; Ruchirawat, S.; Prachayasittikul, V. Mini-Rev. Med. Chem. 2017, 17, 869.
Velihina, Ye.; Scattolin, T.; Bondar, D.; Pil'o, S.; Obernikhina, N.; Kachkovskyi, O.; Semenyuta, I.; Caligiuri, I.; Rizzolio, F.; Brovarets, V.; Karpichev, Ye.; Nolan, St. P. Agents Helv. Chim. Acta 2020, 103, e2000169.
Velihina, Ye. S.; Obernikhina, N. V.; Pilyo, S. G.; Kachkovsky, O. D.; Brovarets, V. S. Curr. Org. Chem. 2021, 25, 1441.
Zhirnov, V. V.; Velihina, Ye. S.; Mitiukhin, O. P.; Brovarets, V. S. Chem. Biol. Drug Des. 2021, 98, 561.
Mortenson, P. N.; Erlanson, D. A.; de Esch, I. J. P.; Jahnke, W.; Johnson, C. N. J. Med. Chem. 2019, 62, 3857.
Ribeiro de Souza Neto, L.; Moreira-Filho, J. T.; Junior Neves, B.; Riveros Maidana, R. L. B.; Ramos Guimarães, A. C.; Furnham, N.; Horta Andrade, C.; Paes Silva, F. Front. Chem. 2020, 8, 93.
Obernikhina, N. V.; Kobzar, O. L.; Kachaeva, M. V.; Kachkovsky, O. D.; Brovarets, V. S. Curr. Comput.-Aided Drug Des. 2022, 18, 95.
Velihina, Y. S.; Obernikhina, N. V.; Pilyo, S. G.; Kachaeva, M. V.; Kachkovsky, O. D. Ukr. Bioorg. Acta 2021, 16(1), 34.
Obernikhina, N.; Zhuravlova, M.; Kachkovsky, O.; Kobzar, O.; Brovarets, V.; Рavlenko, O.; Kulish, M.; Dmytrenko, O. Appl. Nanosci. 2020, 10, 1345.
Marquez, B. L.; Watts, K. S.; Yokochi, A.; Roberts, M. A.; Verdier-Pinard, P.; Jimenez, J. I.; Hamel, E.; Scheuer, P. J.; Gerwick, W. H. J. Nat. Prod. 2002, 65, 866.
Kachaeva, M. V.; Hodyna, D. M.; Semenyuta, I. V.; Pilyo, S. G.; Prokopenko, V. M.; Kovalishyn, V. V.; Metelytsia, L. O.; Brovarets, V. S. Comput. Biol. Chem. 2018, 74, 294.
Jordan, M. Interactions 2010, 17(5), 6.
Obernikhina, N. V.; Nikolaev, R. O.; Kachkovsky, O. D.; Tkachuk, Z. Yu. Dopov. Nac. akad. nauk Ukr. 2019, (6), 75.
Obernikhina, N.; Kachaeva, M.; Shchodryi, V.; Prostota, Ya.; Kachkovsky, O.; Brovarets, V.; Tkachuk, Z. Polycyclic Aromat. Compd. 2020, 40, 1196.
Obernikhina, N.; Pavlenko, O.; Kachkovsky, A.; Brovarets, V. Polycyclic Aromat. Compd. 2021, 41, 2110.
Kachaeva, M. V.; Obernikhina, N. V.; Veligina, E. S.; Zhuravlova, M. Yu.; Prostota, Ya. O.; Kachkovsky, O. D.; Brovarets, V. S. Chem. Heterocycl. Compd. 2019, 55, 448.
Bissantz, C.; Kuhn, B.; Stahl, M. A J. Med. Chem. 2010, 53, 5061.
Rauk, A. Orbital Interaction Theory of Organic Chemistry; John Wiley & Sons: New York, 2001, 2nd ed., p. 34.
Cui, X.; Liu, J.; Xie, L.; Huang, J.; Liu, Q.; Israelachvili, J. N.; Zeng, H. Angew. Chem., Int. Ed. 2018, 57, 11903.
Davis, J. G.; Gierszal, K. P.; Wang, P.; Ben-Amotz, D. Nature 2012, 491, 582.
Berne, B. J.; Weeks, J. D.; Zhou, R. Annu. Rev. Phys. Chem. 2009, 60, 85.
Marx, D. Chem. Phys. Chem. 2006, 7, 1848.
Zheng, S.; Xu, S.; Wang, G.; Tang, Q.; Jiang, X.; Li, Z.; Xu Y.; Wang R.; Lin, F. J. Chem. Inf. Model. 2017, 57, 1535.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams-Young, D.; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery, J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Keith, T.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millam, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox D. J. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, 2016.
Kim, K. S.; Tarakeshwar, P.; Lee, J. Y. Chem. Rev. 2000, 100, 4145.
Churchill, C. D. M.; Rutledge, L. R.; Wetmore, S. D. Phys. Chem. Chem. Phys. 2010, 12, 14515.
Singh, S. K.; Das, A. Phys. Chem. Chem. Phys. 2015, 17, 9596.
Kouza, M.; Banerji, A.; Kolinski, A.; Buhimschi, I.; Kloczkowski, A. Molecules 2018, 23, 1995.
Gao, X.-C.; Hao, Q.; Wang, C.-S. J. Chem. Theory Comput. 2017, 13, 2730.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2022, 58(8/9), 412–420
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Obernikhina, N.V., Kachaeva, M.V., Kachkovsky, O.D. et al. In silico Study of Conjugated Nitrogen Heterocycles Affinity in their Biological Complexes. Chem Heterocycl Comp 58, 412–420 (2022). https://doi.org/10.1007/s10593-022-03107-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-022-03107-5