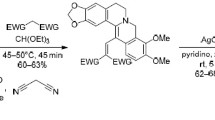
The possibility of obtaining electroneutral substituted 13-vinylberberines was demonstrated experimentally and via quantum-chemical DFT/B3LYP calculations in the 6-31++G(d,p) basis set. The introduction of pharmacophoric fragments conjugated through vinyl moiety opened new possibilities for structural modification of berberine, enabling pronounced changes in tropicity toward supramolecular biological structures. The newly synthesized 13-vinylberberines were stable in their reduced form due to significant intramolecular electron density transfer from berberine ring system to the vinyl moiety bearing electron-withdrawing groups. It was demonstrated that berberine derivatives may exist not only in ion pair form consisting of organic cation and inorganic anion, but also as zwitterionic structures featuring significant intramolecular charge transfer. The obtained 13-vinylberberines exhibited biological activity against the highly pathogenic Vibrio cholerae.


Similar content being viewed by others
References
Nechepureko, I. V.; Salahutdinov, N. F.; Tolstikov, G. A. Khim. Inter. Ust. Razv. 2010, 18(1), 1.
Amin, A. H.; Subbaiah, T. V.; Abbasi, K. M. Can. J. Microbiol. 1969, 15, 1067.
Hwang, B. Y.; Roberts, S. K.; Chadwick, L. R.; Wu, C. D.; Kinghorn, A. D. Planta Med. 2003, 69, 623.
Okunade, A. L.; Hufford, C. D.; Richardson, M. D.; Petterson, J. R.; Clark, A. M. J. Pharm. Sci. 1994, 83, 404.
Basha, S. A.; Mishra, R. K.; Jha, R. N.; Pandey, V. B.; Singh, U. P. Folia Microbiol.(Prague, Czech Repub.) 2002, 47, 161.
Nakamoto, K.; Sadamori, S.; Hamada, T. J. Prosthet. Dent. 1990, 64, 691.
Yamamoto, K.; Takase, H.; Abe, K.; Saitio, Y.; Suzuki, A. Nippon Yakurigaku Zasshi 1993, 101, 169.
Rana, T.; Singh, S.; Kaur, N.; pathania, K.; Farooq, U. Int. J. Pharm. Sci. Rev. Res. 2014, 26(2), 101.
Chen, L.; Bu, Q.; Xu, H.; Liu, Yu.; She, P.; Tan, R.; Wu, Yo. Microbiol. Res. 2016, 186, 44.
Ball, A. R.; Casadei, G.; Samosorn, S.; Bremner, J. B.; Ausubel, F. M.; Moy, T. I.; Lewis, K. ACS Chem. Biol. 2006, 1, 594.
Morita, Yu.; Nakashima, K.-i.; Nishino, K.; Kotani, K.; Tomida, J.; Inoue, M.; Kawamura, Yo. Front. Microbiol. 2016, 7, 1223.
Chu, M.; Xiao, R.-x.; Yin, Yi-n.; Wang, X.; Chu, Zh.-y.; Zhang, M.-b.; Ding, R.; Wang, Yu-d.. Clin. Microbiol.: Open Access 2014, 3, 150.
Liu, Ya.-X.; Xiao, Ch.-L.; Wang, Ya.-X.; Li, Ya.-H.; Yang, Ya.-H.; Li, Ya.-B.; Bi, Ch.-W.; Gao, L.-M.; Jiang, J.-D.; Song, D.-Q. Eur. J. Med. Chem. 2012, 52, 151.
Fan, T.-Yu.; Wang, Ya.-X.; Tang, Sh.; Hu, X.-X.; Zen, Q.-X.; Pang, J.; Yang, Yu.-Sh.; You, Xu.-F.; Song, D.-Q. Eur. J. Med. Chem. 2018, 157, 877.
Demekhin, O. D.; Zagrebaev, A. D.; Burov, O. N.; Kletskii, M. E.; Pavlovich, N. V.; Bereznyak, E. A.; Tsimbalistova, M. V.; Kurbatov, S. V. Chem. Heterocycl. Compd. 2019, 55, 1128.
Elassar, A.-Z. A.; El-Khair, A. A. Tetrahedron 2003, 59, 8463.
Khademi, Z.; Nikoofar, K. RSC Adv. 2020, 10, 30314.
Litvinov, V. P.; Yakunin, Ya. Yu.; Dyachenko, V. D. Chem. Heterocycl Compd. 2001, 37, 37.
Lyamzaev, K. G.; Pustovidko, A. V.; Simonyan, R. A.; Rokitskaya, T. I.; Domnina, L.V.; Ivanova, O. Yu.; Severina, I. I.; Sumbatyan, N. V.; Korshunova, G. A.; Tashlitsky, V. N.; Roginsky, V. A.; Antonenko, Yu. N.; Skulachev, M. V.; Chernyak, B. V.; Skulachev, V. P. Pharm. Res. 2011, 28, 2883.
Burov, O. N.; Kurbatov, S. V.; Morozov, P. G.; Kletskii, M. E.; Tatarov, A. V. Chem. Heterocycl. Compd. 2015, 51, 772.
Becke, A. D. Phys. Rev. A 1988, 38, 3098.
Becke, A. D. J. Chem. Phys. Rev. 1993, 98, 5648.
Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B: Condens. Matter Mater. Phys. 1988, 37, 785.
Burov, О. N.; Kletskii, М. Е.; Gulevskaya, А. V. Russ. Chem. Bull. 2013, 62, 1156.
Kletskii, M. Е.; Burov, О. N.; Dalinger, I. L.; Shevelev, S. А. Comput. Theor. Chem. 2014, 1033, 31.
Simkin, B. Y.; Sheikhet, I. I. Quantum Chemical and Statistical Theory of Solutions: A Computational Approach; Ellis Horwood: London, 1995.
Cances, E.; Mennucci, B.; Tomasi, J. J. Chem. Phys. 1997, 107, 3032.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Montgomery, J. A., Jr.; Vreven, T.; Kudin, K. N.; Burant, J. C.; Millam, J. M.; Iyengar, S. S.; Tomasi, J.; Barone, V.; Mennucci, B.; Cossi, M.; Scalmani, G.; Rega, N.; Petersson, G. A.; Nakatsuji, H.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Klene, M.; Li, X.; Knox, J. E.; Hratchian, H. P.; Cross, J. B.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Ayala, P. Y.; Morokuma, K.; Voth, G. A.; Salvador, P.; Dannenberg, J. J. ; Zakrzewski, V. G.; Dapprich, S.; Daniels, A. D.; Strain, M. C.; Farkas, O.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Ortiz, J. V.; Cui, Q.; Baboul, A. G.; Clifford, S.; Cioslowski, J.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Martin, R. L.; Fox, D. J.; Keith, T.; Al-Laham, M. A.; Peng, C. Y.; Nanayakkara, A.; Challacombe, M.; Gill, P. M. W.; Johnson, B.; Chen, W.; Wong, M. W.; Gonzalez, C.; Pople, J. A. Gaussian 03, Revision E.01; Gaussian, Inc.: Wallingford, 2004.
Schlegel, H. B. Theor. Chim. Acta 1984, 66, 333.
Hirsh, M.; Quapp, W. Chem. Phys. Lett. 2004, 395, 150.
Semina, N. A.; Sidorenko, S. V.; Rezvan, S. P.; Grudinina, S. A.; Strachunskiy, L. S.; Stetsyuk, O. U.; Kozlov, R. S.; Edelshtein, M. V.; Vedmina, E. A.; Stolyarova, L. G.; Vlasova, I. V.; Sereda, Z. S. Klin. Mikrobiol. Antimikrob. Khimioter. 2004, 6(4), 306.
The reported study was funded by the Russian Foundation for Basic Research (RFBR) project number 20-33-90263.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2022, 58(2/3), 144–152
Supplementary Information
ESM 1
(PDF 4712 kb)
Rights and permissions
About this article
Cite this article
Demekhin, O.D., Burov, O.N., Kletskii, M.E. et al. New 13-vinyl derivatives of berberine: synthesis and characterization. Chem Heterocycl Comp 58, 144–152 (2022). https://doi.org/10.1007/s10593-022-03067-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-022-03067-w