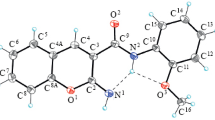
2-(1,3-Benzothiazol-2-yl)-4H-chromen-4-one and spiro[1,4-benzothiazine-2,2'-chromene]-3,4'(3'H,4H)-dione have been synthesized from 4-oxo-4H-chromene-2-carboxylic acid. The course of the reaction that usually occurs between the activated carboxylic acid and aromatic amines was changed upon the use of different reaction conditions (acidic or basic) and 2-aminobenzenethiol. As a result, new heterocyclic derivatives were obtained. Structures of the compounds have been established on the basis of spectral (1D and 2D NMR spectra) and X-ray data.





Similar content being viewed by others
References
The Chemistry of Heterocyclic Compounds: Chromenes, Chromanones, and Chromones; Ellis, G. P., Ed.; John Wiley & Sons: New York, 1977, Vol. 31.
Reis, J.; Gaspar, A.; Milhazes, N.; Borges, F. J. Med. Chem. 2017, 60, 7941.
Gaspar, A.; Matos, M. J.; Garrido, J.; Uriarte, E.; Borges, F. Chem. Rev. 2014, 114, 4960.
Li, N.-G.; Shi, Z.-H.; Tang, Y.-P.; Ma, H.-Y.; Yang, J.-P.; Li, B.-Q.; Wang, Z.-J.; Song, S.-L.; Duan, J.-A. J. Heterocycl. Chem. 2010, 47, 785.
(a) Ghosh, K. C.; Khan, S. Synthesis 1981, 719. (b) Fillion, E.; Dumas, A. M.; Kuropatwa, B. A.; Malhotra, N. R.; Sitler, T. C. J. Org. Chem. 2006, 71, 409.
Valeur, E.; Bradley, M. Chem. Soc. Rev. 2009, 38, 606.
Cagide, F.; Reis, J.; Gaspar, A.; Borges, F. Tetrahedron Lett. 2011, 52, 6446.
(a) Gaspar, A.; Reis, J.; Matos, M. J.; Uriarte, E.; Borges, F. Eur. J. Med. Chem. 2012, 54, 914. (b) Gaspar, A.; Reis, J.; Kachler, S.; Paoletta, S.; Uriarte, E.; Klotz, K.-N.; Moro, S.; Borges, F. Biochem. Pharmacol. 2012, 84, 21. (c) Gaspar, A.; Silva, T.; Yáñez, M.; Vina, D.; Orallo, F.: Ortuso, F.; Uriarte, E.; Alcaro, S.; Borges, F. J. Med. Chem. 2011, 54, 5165. (d) Gaspar, A.; Reis, J.; Fonseca, A.; Milhazes, N.; Viña, D.; Uriarte, E.; Borges, F. Bioorg. Med. Chem. Lett. 2011, 21, 707. (e) Reis, J.; Cagide, F.; Chavarria, D.; Silva, T.; Fernandes, C.; Gaspar, A.; Uriarte, E.; Remião, F.; Alcaro, S.; Ortuso, F.; Borges, F. J. Med. Chem. 2016, 59, 5879. (f) Cagide, F.; Silva, T.; Reis, J.; Gaspar, A.; Borges, F.; Gomes, L. R.; Low, J. N. Chem. Commun. 2015, 51, 2832.
Rigaku CrystalClear SM Expert 2.0 r13; Rigaku Corporation: Tokyo, 2011.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112.
Hübschle, C. B.; Sheldrick, G. M.; Dittrich, B. J. Appl. Crystallogr. 2011, 44, 1281.
Macrae, C. F.; Edgington, P. R.; McCabe, P.; Pidcock, E.; Shields, G. P.; Taylor, R.; Towler, M.; van de Streek, J. J. Appl. Crystallogr. 2006, 39, 453.
Mackay, A. L. Acta Crystallogr., Sect. A: Short Commun. 1984, A40, 165.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2022, 58(1), 68–72
Supplementary Information
ESM 1
(PDF 953 kb)
Rights and permissions
About this article
Cite this article
Cagide, F., Gomes, L.R., Low, J.N. et al. Unexpected conversion of 4-oxo-4H-chromene-2-carboxylic acid to 2-(1,3-benzothiazol-2-yl)-4H-chromen-4-one and spiro[1,4-benzothiazine-2,2'-chromene]-3,4'(3'H,4H)-dione. Chem Heterocycl Comp 58, 68–72 (2022). https://doi.org/10.1007/s10593-022-03058-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-022-03058-x