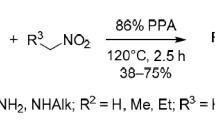
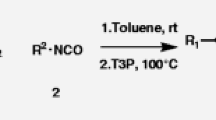
Novel preparative approach for the synthesis of alkylamines with 1,3,4-oxadiazole heterocyclic substituent is described. This method is based on unusual cascade transformation involving formal (4+1) cyclocondensation of hydrazides of amino acids with nitroalkanes electrophilically activated in the presence of polyphosphoric acid.
Similar content being viewed by others

References
Khalilullah, H.; Ahsan, M. J.; Hedaitullah, M.; Khan, S.; Ahmed, B. Mini-Rev. Med. Chem. 2012, 12, 789.
Pangal, A.; Shaikh, J. A. Res. J. Chem. Sci. 2013, 3(12), 79.
Rajak, H.; Kharya, M. D.; Mishra, P. Int. J. Pharm. Sci. Nanotechnol. 2009, 2, 21.
Sun, J.; Makawana, J. A.; Zhu, H.-L. Mini-Rev. Med. Chem. 2013, 13, 1725.
Gurupadaswamy, H. D.; Girish, V.; Kavitha, C. V.; Raghavan, S. C.; Khanum, S. A. Eur. J. Med. Chem. 2013, 63, 536.
Holla, B. S.; Poojary, K. N.; Bhat, K. S.; Ashok, M.; Poojary, B. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 2005, 44B, 1669.
Jisha, M. V.; Kamalabhai Amma, V. K.; Babu, G.; Biju, C. R. J. Chem. Pharm. Res. 2013, 5(6), 64.
Khanam, R.; Ahmad, K.; Hejazi, I. I.; Siddique, I. A.; Kumar, V.; Bhat, A. R.; Azam, A.; Athar, F. Cancer Chemother. Pharmacol. 2017, 80, 1027.
Prasanna Kumar, B. N.; Mohana, K. N.; Mallesha, L.; Veeresh, B. Med. Chem. Res. 2014, 23, 3363.
Rostom, S. A. F.; Shalaby, M. A.; El-Demellawy, M. A. Eur. J. Med. Chem. 2003, 38, 959.
Somani, R. R.; Chiplunkar, S.; Vetale, S. P.; Makhija, D. T.; Shirodkar, P. Y. Int. J. PharmTech Res. 2013, 5, 1233.
Tantak, M. P.; Kumar, A.; Noel, B.; Shah, K.; Kumar, D. ChemMedChem 2013, 8, 1468.
Tantak, M. P.; Malik, M.; Klingler, L.; Olson, Z.; Kumar, A.; Sadana, R.; Kumar, D. Bioorg. Med. Chem. Lett. 2021, 37, 127842.
Vaidya, A.; Pathak, D.; Shah, K. Chem. Biol. Drug Des. 2021, 97, 572.
Zahid, M.; Yasin, K. A.; Akhtar, T.; Hameed, S.; Al-Masoudi, N. A.; Loddo, R.; La Colla, P. ARKIVOC 2009, (xi), 85.
Khan, M.-u.-H.; Akhtar, T.; Al-Masoudi, N. A.; Stoeckli- Evans, H.; Hameed, S. Med. Chem. 2012, 8, 1190.
Kim, R. M.; Rouse, E. A.; Chapman, K. T.; Schleif, W. A.; Olsen, D. B.; Stahlhut, M.; Rutkowski, C. A.; Emini, E. A.; Tata, J. R. Bioorg. Med. Chem. Lett. 2004, 14, 4651.
Li, C.-K.; Ma, Y.-J.; Cao, L.-H. J. Chin. Chem. Soc. (Taipei, Taiwan) 2009, 56, 182.
Syed, T.; Akhtar, T.; Al-Masoudi, N. A.; Jones, P. G.; Hameed, S. J. Enzyme Inhib. Med. Chem. 2011, 26, 668.
Bhati, S.; Kumar, V.; Singh, S.; Singh, J. Lett. Drug Des. Discovery 2020, 17, 1047.
Desai, N. C.; Trivedi, A.; Somani, H.; Jadeja, K. A.; Vaja, D.; Nawale, L.; Khedkar, V. M.; Sarkar, D. Synth. Commun. 2018, 48, 524.
Reddy, G. D.; Park, S.-J.; Cho, H. M.; Kim, T.-J.; Lee, M. E. J. Med. Chem. 2012, 55, 6438.
Guda, D. R.; Park, S.-J.; Lee, M.-W.; Kim, T.-J.; Lee, M. E. Eur. J. Med. Chem. 2013, 62, 84.
Tandon, V. K.; Chhor, R. B. Synth. Commun. 2001, 31, 1727.
Kudelko, A.; Wroblowska, M. Tetrahedron Lett. 2014, 55, 3252.
Aksenov, A. V.; Khamraev, V.; Aksenov, N. A.; Kirilov, N. K.; Domenyuk, D. A.; Zelensky, V. A.; Rubin, M. RSC Adv. 2019, 9, 6636.
Aksenov, N. A.; Arutiunov, N. A.; Kirilov, N. K.; Aksenov, D. A.; Aksenov, A. V.; Rubin, M. Chem. Heterocycl. Compd. 2020, 56, 1067.
Aksenov, N. A.; Aksenov, A. V.; Ovcharov, S. N.; Aksenov, D. A.; Rubin, M. Front. Chem. (Lausanne, Switz.) 2020, 8, 77.
Aksenov, A. V.; Aksenov, N. A.; Nadein, O. N.; Aksenova, I. V. Synlett 2010, 2628.
Aksenov, A. V.; Aksenov, N. A.; Orazova, N. A.; Aksenov, D. A.; Dmitriev, M. V.; Rubin, M. RSC Adv. 2015, 5, 84849.
Aksenov, N. A.; Aksenov, A. V.; Nadein, O. N.; Aksenov, D. A.; Smirnov, A. N.; Rubin, M. RSC Adv. 2015, 5, 71620.
Aksenov, A. V.; Smirnov, A. N.; Aksenov, N. A.; Bijieva, A. S.; Aksenova, I. V.; Rubin, M. Org. Biomol. Chem. 2015, 13, 4289.
Aksenov, A. V.; Aksenov, N. A.; Ovcharov, D. S.; Aksenov, D. A.; Griaznov, G.; Voskressensky, L. G.; Rubin, M. RSC Adv. 2016, 6, 82425.
Aksenov, A. V.; Ovcharov, D. S.; Aksenov, N. A.; Aksenov, D. A.; Nadein, O. N.; Rubin, M. RSC Adv. 2017, 7, 29927.
Aksenov, A. V.; Aksenov, N. A.; Arutiunov, N. A.; Malyuga, V. V.; Ovcharov, S. N.; Rubin, M. RSC Adv. 2019, 9, 39458.
Aksenov, A. V.; Grishin, I. Yu.; Aksenov, N. A.; Malyuga, V. V.; Aksenov, D. A.; Nobi, M. A.; Rubin, M. Molecules 2021, 26, 4274.
Aksenov, N. A.; Aksenov, A. V.; Kirilov, N. K.; Arutiunov, N. A.; Aksenov, D. A.; Maslivetc, V.; Zhao, Z.; Du, L.; Rubin, M.; Kornienko, A. Org. Biomol. Chem. 2020, 18, 6651.
Mondal, M.; Radeva, N.; Fanlo-Virgos, H.; Otto, S.; Klebe, G.; Hirsch, A. K. H. Angew. Chem., Int. Ed. 2016, 55, 9422.
Kudelko, A.; Zielinski, W.; Ejsmont, K. Tetrahedron 2011, 67, 7838.
Brogi, S.; Brindisi, M.; Butini, S.; Kshirsagar, G. U.; Maramai, S.; Chemi, G.; Gemma, S.; Campiani, G.; Novellino, E.; Fiorenzani, P.; Pinassi, J.; Aloisi, A. M.; Gynther, M.; Venskutonyte, R.; Han, L.; Frydenvang, K.; Kastrup, J. S.; Pickering, D. S. J. Med. Chem. 2018, 61, 2124.
Kidwai, M.; Kumar, P.; Goel, Y.; Kumar, K. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 1997, 36B, 175.
This work was supported by the Russian Foundation for Basic Research (grant 20–33–90026) and by the Grants Council of the President of Russian Federation (grant MD-3505.2021.1.3).
Support for the NMR instruments used in this project was provided by the Center of Shared Instrumentation, North Caucasus Federal University (grant 075-15-2021-672).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2022, 58(1), 32–36
Supplementary Information
ESM 1
(PDF 1777 kb)
Rights and permissions
About this article
Cite this article
Aksenov, A.V., Kirilov, N.K., Aksenov, N.A. et al. Electrophilically activated nitroalkanes in the synthesis of substituted 1,3,4-oxadiazoles from amino acid derivatives. Chem Heterocycl Comp 58, 32–36 (2022). https://doi.org/10.1007/s10593-022-03053-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-022-03053-2