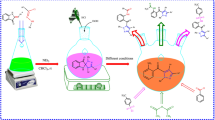
Efficient and concise synthetic protocol to benzo- and pyrazolo-fused medium-sized N,S-heterocycles (e.g,. thiazocinones, thiazoninones, thiazecinones, thiecinones, and thioninones) is developed. The process involves the AlCl3/MeNO2, TfOH or polyphosphoric acid mediated cyclization of pyrazole-based carboxylic acids or esters into tricyclic ketones under mild conditions. The designed protocol offers easy access to biologically and pharmaceutically promising pyrazoles in good yields. The structure elucidation of all new compounds without stereochemical assignments has been carried out by spectral and elemental analysis.


Similar content being viewed by others
References
(a) Koul, A.; Arnoult, E.; Lounis, N.; Guillemont, J.; Andries, K. Nature 2011, 469, 483. (b) Antonow, D.; Thurston, D. E. Chem Rev. 2011, 111, 2815. (c) Dictionary of Alkaloids; Buckingham, J.; Baggaley, K. H.; Roberts, A. D.; Szabó, L. F., Eds.; CRC Press, 2010, 2nd ed. (d) Modern Alkaloids: Structure, Isolation, Synthesis, and Biology; Fattorusso, E.; Taglialatela-Scafati, O. Eds.; Wiley-VCH: Weinheim, 2008. (e) Passler, U. In The Alkaloids; Knolker, H.-J., Ed.; Academic: New York, 2011, Vol. 70, p. 79. (f) Garg, N.; Chandra, T.; Jain, A. B.; Kumar, A. Eur. J. Med. Chem. 2010, 45, 1529. (g) Hall, J. E.; Matlock, J. V.; Ward, J. W.; Gray, K. V.; Clayden, J. Angew. Chem., Int. Ed. 2016, 55, 11153.
(b) Wagman, A. S.; Wentland, M. P. In Comprehensive Medicinal Chemistry II; Taylor, J. B.; Triggle, D. J., Eds.; Elsevier Ltd.: Oxford, 2007, Vol. 7, p. 567. (c) Kresze, G. In Sulfur, Its Significance for Chemistry, for the Geo-, Bio- and Cosmosphere and Technology; Müller, A.; Krebs, B., Eds.; Elsevier: Amsterdam, 1984, Vol. 5, p. 93. (d) Hagiwara, M.; Adachi-Akahane, S.; Nagao, T. Eur. J. Pharmacol. 2003, 466, 63. (e) Arias, H. R.; Targowska-Duda, K. M.; Feuerbach, D.; Sullivan, C. J.; Maciejewski, R.; Jozwiak, K. Neurochem. Int. 2010, 56, 642. (f) Shen, M.; Driver, T. G. Org. Lett. 2008, 10, 3367. (g) Thevis, M.; Opfermann, G.; Schänzer, W. J. Anal. Toxicol. 2003, 27, 53. (h) Tricco, A. C.; Soobiah, C.; Berliner, S.; Ho, J. M.; Ng, C. H.; Ashoor, H. M.; Chen, M. H.; Hemmelgarn, B.; Straus, S. E. CMAJ 2013, 185, 1393. (i) Smith, R; Schwartz, A. N. Engl. J. Med. 1984, 310, 1327. (j) Tatsumi, M.; Groshan, K.; Blakely, R. D.; Richelson, E. Eur. J. Pharmacol. 1997, 340, 249.
(a) Ryabchuk, P.; Matheny, J. P.; Rubina, M.; Rubin, M. Org. Lett. 2016, 18, 6272. (b) Arya, K.; Dandia, A. Bioorg. Med. Chem. Lett. 2008, 28, 114. (c) O'Neil, I. A.; Murray, C. L.; Hunter, R. C.; Kalindjian, S. B.; Jenkins, T. C. Synlett 1997, 75. (d) Kang, G.; Yamagami, M.; Vellalath, S.; Romo, D. Angew. Chem., Int. Ed. 2018, 57, 6527. (e) Majumdar, K. C. RSC Adv. 2011, 1, 1152. (f) Donald, J. R.; Unsworth, W. P. Chem.–Eur. J. 2017, 23, 8780. (g) Li, R.; Farmer, P. S.; Wang, J.; Boyd, R. J.; Cameron, T. S.; Quilliam, M. A.; Walter, J. A.; Howlett, S. E. Drug Des. Discovery 1995, 12, 337. (h) Bariwal, J. B.; Upadhyay, K. D.; Manvar, A. T.; Trivedi, J. C.; Singh, J. S.; Jain, K. S.; Shah, A. K. Eur. J. Med. Chem. 2008, 43, 2279. (i) Gyömöre, Á.; Csámpai, A.; Holzbauer, T.; Czugler, M. Tetrahedron 2011, 67, 2979. (j) Arnold, L. A.; Luo, W.; Guy, R. K. Org Lett. 2004, 6, 3005. (k) Incerti, M.; Acquotti, D.; Sandor, P.; Vicini, P. Tetrahedron 2009, 65, 7487.
Michaut, A.; Rodriguez, J. Angew. Chem., Int. Ed. 2006, 45, 5740.
Limanto, J.; Snapper, M. L. J. Am. Chem. Soc. 2000, 122, 8071.
Ayala, S. L. G.; Stashenko, E.; Palma, A.; Bahsas, A.; Amaro-Luis, J. M. Synlett 2006, 2275.
Ha, H.-J.; Choi, C.-J.; Ahn, Y.-G.; Yun, H.; Dong, Y.; Lee, W. K. J. Org. Chem. 2000, 65, 8384.
Anand, A.; Singh, P.; Mehra, V.; Amandeep; Kumar, V.; Mahajan, M. P. Tetrahedron Lett. 2012, 53, 2417.
Upadhayaya, R. S.; Lahore, S. V.; Sayyed, A. Y.; Dixit, S. S.; Shinde, P. D.; Chattopadhyaya, J. Org. Biomol. Chem. 2010, 8, 2180.
Ylijoki, K. E. O.; Stryker, J. M. Chem. Rev. 2012, 113, 2244.
(a) Krause, N.; Winter, C. Chem. Rev. 2011, 111, 1994. (b) Müller, T. J. Synthesis 2012, 159.
Goswami, P.; Borah, A. J.; Phukan, P. J. Org. Chem. 2014, 80, 438.
Curran, D. P.; Porter, N. A.; Giese, B.; Eliel, E. L. Stereochemistry of Radical Reactions: Concepts, Guidelines, and Synthetic Applications; VCH: New York, 1996.
Basavaiah, D.; Reddy, G. C. ARKIVOC 2016, (ii), 172.
Tietze, L. F.; Brasche, G.; Gericke, K. M. Domino Reactions in Organic Synthesis; Wiley-VCH: Weinheim, 2006.
Ihara, M. Chem. Pharm. Bull. 2006, 54, 765.
(a) Ried, W.; Marx, W. Chem. Ber. 1957, 90, 2683. (b) Khatik, G. L.; Kumar, R.; Chakraborti, A. K. Synthesis 2007, 541. (c) Shcherbakova, I. In Comprehensive Heterocyclic Chemistry III; Katritzky, A. R.; Ramsden, C. A.; Scriven, E. F. V.; Taylor, R. J. K., Eds.; Pergamon Press: New York, 2008, Vol. 14, p. 255. (d) Calvo, L. A.; Gonzalez-Ortega, A.; Marcos, R.; Perez, R. M.; Sanudo, M. C. Tetrahedron 2008, 64, 3691. (e) Levai; Jeko, J. ARKIVOC 2008, (xvii), 234.
(a) Paquette, L. A.; Barton, W. R. S.; Gallucci, J. C. Org. Lett. 2004, 6, 1313. (b) Karsch, S.; Freitag, D.; Schwab, P.; Metz, P. Synthesis 2004, 1696. (c) Chihab-Eddine, A.; Daich, A.; Jilale, A.; Decroix, B. J. Heterocycl. Chem. 2000, 37, 1543. (d) Freitag, D.; Schwab, P.; Metz, P. Tetrahedron Lett. 2004, 45, 3589.
(a) Alvarez, M.; Joule, J. A. In The Alkaloids: Chemistry and Biology; Cordell, G. A., Ed.; Academic Press: New York, 2001, Vol. 57, p. 235. (b) Alper, K. R.; Lotsof, H. S.; Kaplan, C. D. J. Ethnopharmacol. 2008, 115, 9. (c) Baumann, M. H.; Rothman, R. B.; Pablo, J. P.; Mash, D. C. J. Pharmacol. Exp. Ther. 2001, 297, 531. (d) Noguchi, Y.; Hirose, T.; Furuya, Y.; Ishiyama, A.; Otoguro, K.; Omura, S.; Sunazuka, T. Tetrahedron Lett. 2012, 53, 1802.
Eliel, E. L.; Wilen, S. H. Stereochemistry of Organic Compounds; Wiley: New York, 1994.
(a) Parenty, A.; Moreau, X.; Campagne, J. M. Chem. Rev. 2006, 106, 911. (b) Kobayashi, Y.; Asano, M.; Yoshida, S.; Takeuchi, A. Org. Lett. 2005, 7, 1533. (c) Buszek, K. R.; Jeong, Y.; Sato, N.; Still, P. C.; Muiño, P. L.; Ghosh, I. Synth. Commun. 2001, 31, 1781. (d) Nagata, T.; Nakagawa. M.; Nishida, A. J. Am. Chem. Soc. 2003, 125, 7484. (e) Shiina, I.; Kubota, M.; Ibuka, R. Tetrahedron Lett. 2002, 43, 7535. (f) Petri, A. F.; Bayer, A.; Maier, M. E. Angew. Chem., Int. Ed. 2004, 43, 5821. (g) Trost, B. M.; Chisholm, J. D. Org. Lett. 2002, 4, 3743. (h) Marcantoni, E.; Massaccesi, M.; Petrini, M.; Bartoli, G.; Bellucci, M. C.; Bosco, M.; Sambri, L. J. Org. Chem. 2000, 65, 4553.
Bates, D. K.; Li, X.; Jog, P. V. J. Org. Chem. 2004, 69, 2750.
Lu, S. M.; Alper, H. J. Am. Chem. Soc. 2005, 127, 14776.
Pei, Y.; Lilly, M. J.; Owen, D. J.; D'Souza, L. J.; Tang, X. Q.; Yu, J.; Nazarbaghi, R.; Hunter, A.; Anderson, C. M.; Glasco, S.; Ede, N. J.; James, I. W.; Maitra, U.; Chandrasekaran, S.; Moos, W. H.; Ghosh, S. S. J. Org. Chem. 2003, 68, 92.
Mukherjee, C.; Biehl, E. Heterocycles 2004, 63, 2309.
Doxsee, K. M. In Comprehensive Heterocyclic Chemistry II; Katritzky, A. R.; Rees, C. W.; Scriven, E. F. V., Eds.; Pergamon: Oxford, 1996, Vol. 9, p. 527.
Griesbeck, A. G.; Oelgemöller, M.; Lex, J.; Haeuseler, A.; Schmittel, M. Eur. J. Org. Chem. 2001, 1831.
Bates, D. K.; Li, K. J. Org. Chem. 2002, 67, 8662.
Moormann, A. E.; Metz, S.; Toth, M. V.; Moore, W. M.; Jerome, G.; Kormeier, C.; Manning, P.; Hansen, D. W.; Pitzele, B. S.; Webber, R. K. Bioorg. Med. Chem. Lett. 2001, 11, 2651.
Maraccini, S.; Miguel, D.; Torroba, T.; Garcìa-Valverde, M., J. Org. Chem. 2003, 68, 3315.
Campiani, G.; Nacci, V.; Fiorini, I.; De Filippis, M. P.; Garofano, A.; Ciani, S. M.; Greco, G.; Novellino, E.; Manzoni, C.; Pennini, T. Eur. J. Med. Chem. 1997, 32, 241.
Kuti, M.; Rábai, J.; Kapovits, I.; Jalsovszky, I.; Argay, G.; Kálmán, A.; Párkányi, L. J. Mol. Struct. 1996, 382, 1
Federsel, H. J.; Glassare, G.; Högström, K.; Wiestal, J.; Zinko, B.; Odman, C. J. Org. Chem. 1995, 60, 2597.
Sashida, H.; Tsuchiya, T. Heterocycles 1984, 22, 1303.
Manhas, M. S.; Amin, S. G.; Bose, A. K. Heterocycles 1976, 5, 669.
(a) Barclay, L. R. C. In Friedel–Crafts and Related Reactions; Olah, G. A., Ed.; Wiley Interscience: New York, 1964, Vol. II, Chap. 22, p. 786. (b) Ross, J.; Xiao, J. L. Green Chem. 2002, 4, 129. (c) Simone, F. D.; Andres, J.; Torosantucci, R.; Waser, J. Org. Lett. 2009, 11, 1023. (d) Stang, E. M.; White, M. C. J. Am. Chem. Soc. 2011, 133, 14892. (e) Li, S.; Chiu, P. Tetrahedron Lett. 2008, 49, 1741. (f) Jorgensen, K. A. Synthesis 2003, 1117. (g) MacMillan, D. W. C.; Overman, L. E. J. Am. Chem. Soc. 1995, 117, 10391. (h) Zhao, Y.-L.; Lou, Q.-X.; Wang, L.-S.; Hu, W.-H.; Zhao, J.-L. Angew. Chem., Int. Ed. 2017, 56, 338. (i) Pilli, R. A.; Victor, M. M. Tetrahedron Lett. 1998, 39, 4421. (j) Fürstner, A. Top. Catal. 1997, 4, 285. (k) Anand, R. V.; Baktharaman, S.; Singh, V. K. J. Org. Chem. 2003, 68, 3356. (l) Wales, S. M.; Walker, M. M.; Johnson, J. S. Org. Lett. 2013, 15, 2558.
Galli, C.; Mandolini, L. Eur. J. Org. Chem. 2000, 3117.
(a) Katritzky, A. R.; Xu, Y.-J.; He, H.-Y. J. Chem. Soc., Perkin Trans. 1 2002, 592. (b) Cul, A.; Daich, A.; Decroix, B.; Sanz, G.; Van Hijfte, L. Heterocycles 2004, 64, 33. (c) Mamouni, A.; Daich, A.; Decroix, B. Synth. Commun. 1997, 27, 2241. (d) Pigeon, P.; Decroix, B. J. Heterocycl. Chem. 1997, 34, 375. (e) Oniciu, D. C. In Comprehensive Heterocyclic Chemistry III; Katritzky, A. R.; Ramsden, C. A.; Scriven, E. F. V.; Taylor, R. J. K., Eds.; Pergamon Press: New York, 2008, Vol. 14, p. 1. (f) Chen, L.; Zhou, F.; Shi, T.-D.; Zhou, J. J. Org. Chem. 2012, 77, 4354. (g) Netchitailo, P.; Othman, M.; Decroix, B. J. Heterocycl. Chem. 1997, 34, 321.
(a) Abd El-Aal, H. A. K.; Khalaf, A. A. Aust. J. Chem. 2019, 72, 276. (b) Abd El-Aal, H. A. K. ARKIVOC 2018, (iii), 45. (c) Abd El-Aal, H. A. K. Aust. J. Chem. 2017, 70, 1082. (d) Abd El-Aal, H. A. K.; Khalaf, A. A. ARKIVOC 2019, (v), 265. (e) Abd El-Aal, H. A. K.; Khalaf, A. A. ARKIVOC 2013, (iv), 306. (f) Abd El-Aal, H. A. K.; Khalaf, A. A. Chem. Heterocycl. Compd. 2019, 55, 632. [Khim. Geterotsikl. Soedin. 2019, 55, 632.] (g) Abd El-Aal, H. A. K.; Khalaf, A. A.; El-Khawaga, A. M. A. J. Heterocycl. Chem. 2014, 51, 262. (h) Abd El-Aal, H. A. K. ARKIVOC 2015, (v), 230.
(a) Olah, G. A.; Krishnamurti, R.; Surya Prakash, G. K. In Comprehensive Organic Synthesis; Trost, B. M.; Fleming, I., Eds.; Pergamon Press: Oxford, 1991, Vol. 3, p. 293. (b) Roberts, R. M.; Khalaf, A. A. Friedel–Crafts Chemistry: A Century of Discovery; Marcel Dekker: New York, 1984. (c) Olah, G. A. In Friedel–Crafts Chemistry; Olah, G. A., Ed.; Wiley: New York, 1973. (d) Bandini, M.; Melloni, A.; Tommasi, S.; Umani-Ronchi, A. Synlett 2005, 1199. (e) Terrasson, V.; Marcia de Figueiredo, R.; Campagne, J. M. Eur. J. Org. Chem. 2010, 14, 2635. (f) Poulsen, T. B.; Jørgensen, K. A. Chem. Rev. 2008, 108, 2903.
(a) Elguero, J. In Comprehensive Heterocyclic Chemistry II; Katritzky, A. R.; Rees, C. W.; Scriven, E. F. V., Eds.; Elsevier: Oxford, 1996, Vol. 3, p. 1. (b) Brahmbhatt, G. C.; Sutariya, T. R.; Atara, H. D.; Parmar, N. J.; Gupta, V. K.; Lagunes, I.; Padrón, J. M.; Murumkar, P. R.; Yadav, M. R. Mol. Diversity 2020, 24, 355.
Dauben, W. G.; Tilles, H. J. Am. Chem. Soc. 1950, 72, 3185.
(a) Kakushima, M.; Hamel, P.; Frenette, R.; Rokach, J. J. Org. Chem. 1983, 48, 3214. (b) Carey, F. A.; Sundberg, R. J. In Advanced Organic Chemistry. Part B: Reactions and Synthesis; Kluwer Academic/Plenum Publishers: New York, 2001, 4th ed. (c) Olah, G. A.; Kobayashi, S. J. Am. Chem. Soc. 1971, 93, 6994. (d) Huffman, J. W.; Smith, V. J.; Padgett, L. W. Tetrahedron 2008, 64, 2104. (e) Tan, L. K.; Brownstein, S. J. Org. Chem. 1983, 48, 302. (f) Golebiewski, W.; Marek; Gucma, M. Synthesis 2007, 3599.
Choi, S.; Brown, H. C. J. Am. Chem. Soc. 1963, 85, 2596.
March, J. Advanced Organic Chemistry; John Wiley and Sons: New York, 1999–2000, 4th ed.
The author is grateful for all the facilities received while performing and writing this work by the Chemistry department, Faculty of science Assiut University, Assiut, Egypt.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2020, 56(10), 1353–1362
Supplementary Information
ESM 1
(PDF 8486 kb)
Rights and permissions
About this article
Cite this article
Abd El-Aal, H.A.K. Target-oriented synthesis of functionalized pyrazolo-fused medium-sized N,S-heterocycles via Friedel–Crafts ring closure approach. Chem Heterocycl Comp 56, 1353–1362 (2020). https://doi.org/10.1007/s10593-020-02822-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-020-02822-1